INTRODUCTION
The North American Great Lakes region, defined here as the area within the Great Lakes drainage basin, was completely glaciated during the most recent periods of Pleistocene glaciation (Dorr and Eschman 1970; Williams et al. 1998). In geologic terms, therefore, the contemporary flora of the region is young, having assembled only within the last 10,000 to 15,000 years. Most of the native vascular flora—ca. 2,500 species (Peirson 2010)—consists of species that migrated into the region following glaciation and that inhabit basic vegetation formations within the region. However, approximately 60 endemic vascular plant taxa have been described from the region. Unlike most widespread members of the flora, many of these endemics are restricted to ecologically specialized habitats, often along the shores of the Great Lakes (Peirson 2010).
The three best known Great Lakes shoreline endemics are Iris lacustris Nutt. (dwarf lake iris), Solidago houghtonii Torr. & A. Gray (Houghton’s goldenrod), and Cirsium pitcheri Torr. & A. Gray (Pitcher’s thistle), all of which are federally-listed threatened species. Iris lacustris and S. houghtonii both have geographic distributions that are tightly centered around the Straits of Mackinac in northern Michigan, a general distribution that is shared by a number of Great Lakes endemics. Iris lacustris is restricted to calcareous shores and alvar habitats of the Niagara Escarpment from eastern Wisconsin and northern Michigan to the Bruce Peninsula in Ontario (Guire and Voss 1963; Trick and Fewless 1984), while S. houghtonii is likewise essentially confined to sandy and rocky shores from northern Michigan along the northern edge of Lake Huron to the Bruce Peninsula (Guire and Voss 1963; Morton 1979; Morton and Venn 2000; Laureto and Barkman 2011). The distribution of Cirsium pitcheri, while still encompassing the Straits of Mackinac, is broader than those of the previous two species. Pitcher’s thistle inhabits beaches and active sand dunes from the southern shores of Lake Michigan north through Lake Huron, with several populations along the Lake Superior shoreline as well (Guire and Voss 1963; Voss 1996). Taken together, these three endemics illustrate the characteristic ecogeographic patterns found in a number of Great Lakes shoreline endemics (Peirson 2010).
Another striking Greats Lakes shoreline endemic is Gillman’s goldenrod or dune goldenrod, treated here at the species level as Solidago gillmanii (A. Gray) Steele (following Semple and Peirson 2013). Like Cirsium pitcheri, this species is a characteristic component of sand dune vegetation along the shores of Lake Michigan and northern Lake Huron. Gray (1882) originally described this goldenrod (at the time as S. humilis Pursh var. gillmanii A. Gray), as occurring on “sand hillocks on the shores of Lakes Superior and Michigan.” Voss (1996) likewise included dune goldenrods along the southern shore of Lake Superior in S. gillmanii (at the time as S. simplex Kunth var. gillmanii (A. Gray) Ringius), even using a photograph of a particularly robust Lake Superior plant from Chippewa County, Michigan in his Plate 7F. However, during fieldwork to sample populations of S. gillmanii near Deer Park, Luce County, Michigan, as part of a broader phylogeographic study of the species and other members of Solidago subsect. Humiles (Rydb.) Semple, it became apparent that these Lake Superior dune goldenrods did not fit well within S. gillmanii from the dunes of Lakes Michigan and Huron. While the plants shared the overall form and apparent sand dune adaptations of S. gillmanii, the Lake Superior plants had conspicuous pubescence on both the leaves and the stems, a characteristic not found in S. gillmanii. They also lacked the glutinous (sticky) leaves and capitula (flower heads) of S. gillmanii. It was clear that these dune goldenrods warranted a closer look.
The major objectives of this study were (1) to use field and herbarium studies to examine the Lake Superior dune goldenrod and describe its distribution and ecology; (2) to compare this dune goldenrod to other goldenrods in the Great Lakes region and to assess its taxonomic placement; (3) to use flow cytometry to determine its ploidy; and (4) to use the information thereby collected to place it more broadly in the context of the endemic flora of the region.
MATERIALS AND METHODS
Study System—
Solidago L. (Asteraceae: Astereae) is a genus of over 130 species of perennial herbs, approximately 120 of which are native to North America (Semple and Cook 2006; Semple 2022). Michigan is home to 27 currently recognized species of goldenrod (as summarized from Voss and Reznicek 2012; Semple and Peirson 2013; Semple et al. 2017a). The most recent classifications of the genus have recognized four subgenera, 15 sections, and 12 subsections, based on morphology and a polygenomic DNA phylogeny (Semple and Beck 2021; Semple et al. 2022, submitted). To date, no comprehensive molecular phylogenetic framework has been published for Solidago. The genus is well known for its complex patterns of infraspecific cytogeographic variation, with approximately 46% of recognized species showing some incidence of polyploidy in their histories (Peirson et al. 2012).
Goldenrods are characteristic members of the late summer- and fall-blooming floras across much of North America. Their most commonly yellow-rayed heads are arranged into conspicuous capitulescences in many species and can be quite showy. Goldenrods are self-incompatible and are pollinated by a variety of insect pollinators (Gross and Werner 1983; Havercamp and Whitney 1983). Seed dispersal in Solidago species is by wind; the cypselae have a bristly pappus that aids in wind dispersal (Hood and Semple 2003).
Field Investigations—
Fieldwork was conducted along the southern Lake Superior shoreline in Michigan. Sand dune and shoreline communities were surveyed from Marquette in Marquette County to Whitefish Point in Chippewa County. Populations of the Lake Superior dune goldenrod were located and sampled along a 75-km shoreline transect from Superior Campground Beach east of Grand Marais to Whitefish Point. Locality and voucher information is presented in Table 1. In addition to the target species, individuals of S. hispida Muhl. from the southern shore of Lake Superior were also sampled for comparison. At each site, rhizome cuttings from widely spaced individuals (clones spaced > 3 m apart) were harvested in the field and transplanted to Matthaei Botanical Gardens at the University of Michigan. The cuttings, consisting of a rosette of leaves and approximately four cm of rhizome with multiple nodes and buds, were potted in standard potting soil. Voucher specimens were harvested in the field, or taken from greenhouse-grown plants if not flowering in the field, and deposited in the University of Michigan Herbarium (MICH).
Locality and voucher information for populations of Lake Superior dune goldenrod and S. hispida sampled for flow cytometry analyses. All populations were in Michigan, U.S.A. Vouchers are deposited at MICH.
| Taxon Population | County | Latitude | Longitude | Voucher |
|---|---|---|---|---|
| S. hispida var. hispida | ||||
| Au Train Bay | Alger | 46.43 | −86.83 | Peirson 853 |
| Superior Campground Beach | Luce | 46.68 | −85.75 | Peirson 856 |
| S. hispida var. huronensis | ||||
| Great Sand Bay | Keweenaw | 47.45 | −88.22 | Peirson 627 |
| Lake Superior dune goldenrod | ||||
| East of Deer Park | Luce | 46.68 | −85.61 | Peirson 861 |
| East of 3-Mile Creek | Luce | 46.73 | −85.32 | Peirson 833 |
| Superior Campground Beach | Luce | 46.68 | −85.75 | Peirson 855 |
| West of Whitefish Point | Chippewa | 46.79 | −84.99 | Peirson 857 |
Herbarium Investigations—
To determine the full geographic range of the species and to compare it to other sand dune endemic goldenrods in the Great Lakes region, Solidago specimens from GH, MICH, MO, MSC, MT, TEX, and UMBS were studied.
DNA Ploidy Determination—
DNA ploidy (sensu Hiddeman et al. 1984) was determined by flow cytometry after the relative DNA content (from flow cytometry) was calibrated with chromosome counts and flow cytometry determinations from other studies (see below). At least one calibration/standardization was used for each recovered DNA ploidy level (2x and 4x). Similar methods have been used successfully for other species of Solidago (Halverson et al. 2008; Schlaepfer et al. 2008; Laureto and Pringle 2010; Peirson et al. 2012).
Methods follow those described in Peirson et al. (2012). Fresh Solidago leaf material was harvested from greenhouse-grown plants and stored in cool conditions for up to one week. For each sample, approximately one half of a young leaf was chopped with a clean razor blade in 0.8 ml ice-cold LB01 buffer (Doležel et al. 1989) with 50 µg/ml propidium iodide and 50 µg/ml RNAse added. An approximately equal amount of fresh leaf from Glycine max (L.) Merr. ‘Polanka’ was co-chopped as an internal DNA content standard (2.5 pg/2c; cited in Doležel et al. 1994; Doležel et al. 2007). After chopping, each sample was filtered through a 30-µm filter into a microcentrifuge tube. Filtered samples were then centrifuged. The supernatant was subsequently removed, and the pellet was resuspended in 50 µg/ml propidium iodide and incubated at room temperature for 20–45 minutes. Samples were run on a BD FACSCalibur flow cytometer in the Department of Integrative Biology at the University of Guelph. Samples were run at medium pressure for 90 seconds, and data were acquired using CellQuest Pro software (BD Biosciences).
Samples were analyzed using Modfit (Verity Software) to estimate peak means, CVs (coefficients of variation), and nuclei number. DNA content was calculated as:
where 2.5 equals the standardized mean genome size of Glycine max (in pg/2C) and the other mean values represent the experimentally determined values for each sample and where pg/2C is the mean nuclear DNA content in picograms expressed on a diploid basis.
RESULTS AND DISCUSSION
Study of herbarium specimens from dune systems along the southern shore of Lake Superior supported the preliminary conclusion based on initial field observations that the goldenrods there differed from Solidago gillmanii and likely did not belong to Solidago subsect. Humiles, to which S. gillmanii belongs. The Lake Superior dune goldenrods shared the overall form and apparent sand dune adaptations of S. gillmanii (e.g., presence of elongate vertical rhizomes that allow survival from sand burial) but differed noticeably in their pubescent stems and foliage. Vegetative pubescence (outside of the floral arrays) is not found in S. gillmanii or the other members of subsect. Humiles. Subsequent fieldwork along the southern shore of Lake Superior confirmed the earlier observations. Plants in these populations were consistently pubescent (rarely only sparsely so) and were also not noticeably glutinous. Sticky leaves, stems, and capitula have been used as defining characteristics of members of subsect. Humiles sensu lato and are readily apparent in S. gillmanii in the field.
The vegetative pubescence and virgate (wand-like) inflorescences of the Lake Superior dune goldenrods suggested a possible relationship with Solidago hispida Muhl. (hairy goldenrod). That species is widespread throughout eastern North America, extending as far west as the Canadian prairie provinces, in a variety of dry, often sandy or rocky habitats (Semple et al. 2017b). The typical pubescent form of S. hispida occurs throughout Michigan. In the Lake Superior region, it occurs along the shore in open, sandy woods, on lakeshore bluffs, and in rock outcrop habitats, but is almost never present on the open dunes (except occasionally at the margins in more stabilized areas). Examination of plants in the field and the herbarium revealed that typical S. hispida seems to lack the elongate vertical rhizomes that would allow for survival from sand burial in open dune habitats. While sharing vegetative pubescence and virgate inflorescences, the Lake Superior dune goldenrods differed morphologically from nearby, typical S. hispida in their larger stature, clump-forming habit of several to many stems, elongate vertical rhizomes, and in their larger capitula (ca 6.5 mm vs ca 4.5 mm long, respectively). These observations supported the idea that these dune goldenrods along the southern shore of Lake Superior represented an undescribed species that was possibly aligned with S. hispida and subsect. Erectae (G. Don) Semple & J. B. Beck.
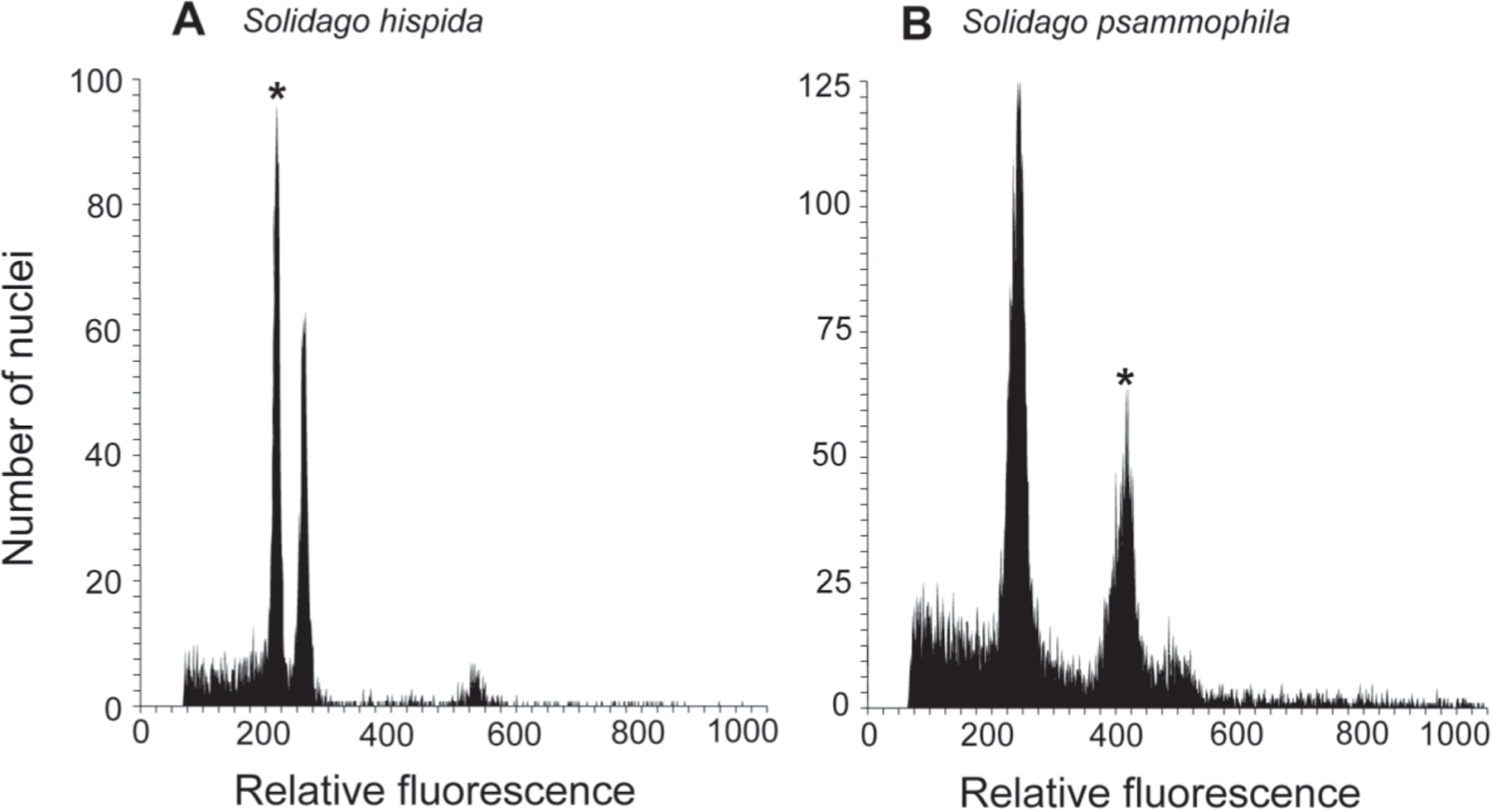
In some Solidago complexes, ecogeographic separation and/or morphological differences (e.g., the size of the capitula) have also been associated with differences in ploidy level (as discussed in Peirson et al. 2012). Given the observed habitat and morphological differences between the dune goldenrods, hereafter referred to S. psammophila, and nearby S. hispida, this study also examined if there were differences in ploidy as well. DNA ploidy determinations from flow cytometry were obtained for 71 individuals from four populations of S. psammophila and for 26 individuals from three populations of S. hispida from the Lake Superior shore (Tables 1 and 2). Flow cytometry recovered two non-overlapping DNA ploidy groups that correspond to diploid (2x = 18) and tetraploid (4x = 36) individuals (Table 2; Figure 1). All sampled individuals of S. psammophila were found to be tetraploid, whereas individuals of S. hispida were found to be uniformly diploid. The 2x and 4x DNA ploidy groupings from this study are consistent with groupings found in previous flow cytometry studies of other Solidago species (e.g., Halverson et al. 2008; Schlaepfer et al. 2008; Peirson et al. 2012). The diploid determination for S. hispida is also consistent with previous cytological studies of the species (as summarized from Semple and Cook 2006; Semple et al. 2017b). Solidago psammophila would be the second tetraploid in the S. bicolor–S. hispida complex, after the upper Midwest endemic S. sciaphila Steele (following Semple et al. 2017b). Whether S. psammophila formed through chromosome doubling within a single S. hispida-like ancestral species (autopolyploidy) or as a result of hybridization and subsequent chromosome doubling (allopolyploidy) was not examined in this study. The narrowly circumscribed distribution along a short portion of the southern shore of Lake Superior suggests a single origin of S. psammophila.
Representative fluorescence histograms of stained nuclei isolated during flow cytometry analyses of fresh tissue of Solidago psammophila, S. hispida, and the internal standard (Glycine max ‘Polanka’). The Solidago peak is indicated by an asterisk (*). (A) diploid S. hispida from Superior Campground Beach, Luce County, Michigan; (B) tetraploid S. psammophila from Deer Park, Luce County, Michigan.
DNA content and DNA ploidy as determined by flow cytometry analysis of fresh leaf tissue from Solidago psammophila and S. hispida.
| Taxon Population | No. Indiv. | DNA Ploidy | DNA Content (pg/2C) | ||
|---|---|---|---|---|---|
| Mean (± SD) | Min. | Max. | |||
| S. hispida var. hispida | |||||
| Au Train Bay | 19 | 2x | 2.09 (0.03) | 2.04 | 2.15 |
| Superior Campground Beach | 5 | 2x | 2.07 (0.01) | 2.06 | 2.09 |
| S. hispida var. huronensis | |||||
| Great Sand Bay | 2 | 2x | 2.12 (0.01) | 2.12 | 2.13 |
| S. psammophila | |||||
| East of Deer Park | 31 | 4x | 4.37 (0.11) | 4.16 | 4.60 |
| East of 3-Mile Creek | 5 | 4x | 4.27 (0.07) | 4.19 | 4.36 |
| Superior Campground Beach | 21 | 4x | 4.36 (0.07) | 4.22 | 4.49 |
| West of Whitefish Point | 14 | 4x | 4.26 (0.08) | 4.16 | 4.40 |
Given that Gray’s (1882) original concept of Solidago humilis var. gillmanii was mixed and included both the Lake Michigan/Lake Huron dune plants and the Lake Superior S. psammophila, scrutiny of the original description and the type of S. gillmanii was necessary. Gray stated in his description that S. humilis var. gillmanii was “an extreme form of this variable species, with dentate even laciniate leaves and an open compound panicle; growing on sand hillocks on the shores of Lakes Superior and Michigan.” Gray, however, did not cite any collections or designate a type for var. gillmanii. As follow-up to his biosystematic study of the “S. spathulata–S. glutinosa complex,” which included S. gillmanii, Ringius (1987) reviewed the nomenclature of the group and designated a number of lectotypes, including for S. humilis var. gillmanii. Ringius’ choice of lectotype, at first glance, presented a bit of a quandary. He stated that there were three collections from “the south shore of Lake Superior … sent to A. Gray by W. Boott” and identified by Gray as var. gillmanii that could serve as candidates for the lectotype (two 1875 collections (GH, NY) and a single 1879 collection (GH)). Ultimately, Ringius designated the 1879 GH collection as lectotype and stated that it matched “the protologue in having laciniate leaf margins and an open compound panicle.” Examination of the 1875 and 1879 collections, however, revealed that the 1879 collection designated as lectotype by Ringius was not a plant from the Lake Superior shore.
The labels on the 1875 GH collection indicate “s. Shor l. Superior root sent by W. Boott.” Examination of that collection, which consists of two sheets with portions of the same rosette (GH-00274538, GH-00274539), revealed sparse pubescence on the rosette leaves as well as capitula that did not appear glutinous. Similarly, examination of a digital image of the NY collection (NY-02369465) also appears to show pubescence on the rosette leaves and an overall lack of glutinosity. The pubescence on the foliage, the lack of glutinosity, and the label data indicating that the collections originated from the southern shore of Lake Superior confirm that the 1875 collections are Solidago psammophila. The label on the lectotype, the 1879 GH collection (00012486), indicates “Roots from upper Michigan by W. Boott” and “same as 1875.” Examination of the lectotype did not reveal any vegetative pubescence outside of the floral array. In addition, the capitula and leaves within the capitulescence appear to have been glutinous (e.g., they have a slightly varnished/resinous appearance). Morphologically, the 1879 collection is a characteristic, cultivated specimen of S. gillmanii, consistent with plants cultivated from Lake Michigan dune systems at the Matthaei Botanical Gardens during this and previous studies. Ringius (1987) stated that the 1879 GH collection was from the south shore of Lake Superior, presumably inferring that the notation on the label of “same as 1875” indicated that the specimen was from the same plant. Examination of the collections revealed that this cannot be correct, since the 1875 and 1879 collections represent S. psammophila and S. gillmanii, respectively. By “same as 1875” Gray presumably simply meant that he considered the collections to represent the same taxon. In addition, the locality information of “upper Michigan” on the label would indicate that the collection came from Michigan’s Upper Peninsula, not specifically that it came from the Lake Superior shore, and Gray did include “Lake Michigan” in the original description. The lectotype of S. gillmanii originated from the dunes along northern Lake Michigan/Huron in Michigan’s Upper Peninsula. Solidago gillmanii is quite common on the dunes there and absent from the Lake Superior shore, so far as is known.
TAXONOMIC TREATMENT
Solidago psammophila J.A. Peirson, sp. nov.—-TYPE: U.S.A. Michigan: Alger Co., Sect. 7, ca. 6 miles west of Grand Marais, high dunes above Lake Superior, July 26, 1948, McVaugh 9586 (holotype: MICH!, isotypes: MT!, UMBS!).
Perennial herbs from branching vertical rhizomes or caudices. Stems 1 to ca. 15, 35–75 cm, ascending or more commonly erect (occasionally slightly decumbent at base), generally unbranched below the capitulescence, sparsely to densely hispid or short-villous proximally, occasionally appearing glabrate, especially if trichomes have been abraded by blowing sand, moderately to densely hispid to strigose in the capitulescence. Leaves alternate, simple, petiolate or sessile, sometimes stipitate glandular (but not becoming resinous or glutinous), moderately or sparsely short-pubescent to sericeous or more rarely strigose. Basal rosette and proximal stem leaves petiolate, petiole ciliate, blade ovate-oblanceolate to narrowly oblanceolate, tapering to petiole, 4–14 cm long, 0.7–3 cm wide, apex acute to obtuse or less often rounded, margin serrate. Mid and distal stem leaves sessile, lanceolate to linear, 1–4 cm long, 0.2–0.6 cm wide, reduced upward, margin entire or sparsely serrate. Capitulescence narrowly to broadly elongate-paniculiform, 7–25 cm long, 2.5–8.5 cm wide, consisting of short axillary and terminal racemiform clusters, lower branches occasionally elongated in larger plants, branches strigulose; heads few to numerous, not secund. Peduncles 3–10 mm long, strigulose; bracteoles few, linear. Involucres campanulate, 5–8.1 mm long. Phyllaries in 3–4 graduated rows, the outer ones ovate, the inner ones linear-oblong, apex acute to obtuse or rounded, often ciliate or fringed. Ray florets 8–13, strap-shaped, 2–3 mm long, 0.6–0.9 mm wide. Disc florets 8–15, corollas 3.5–5 mm long. Cypselae narrowly obconic, antrorse-strigose, ca. 3 mm long. 2n = 36 (from DNA ploidy determination). (Figure 2).
Etymology.
The specific epithet psammophila, which means sand-loving, refers to the restricted ecological distribution of the species in open dune habitats.
Phenology.
Plants generally begin flowering in early August and continue until late September. Cypselae mature and are dispersed from mid-September through October.
Distribution and Ecology.
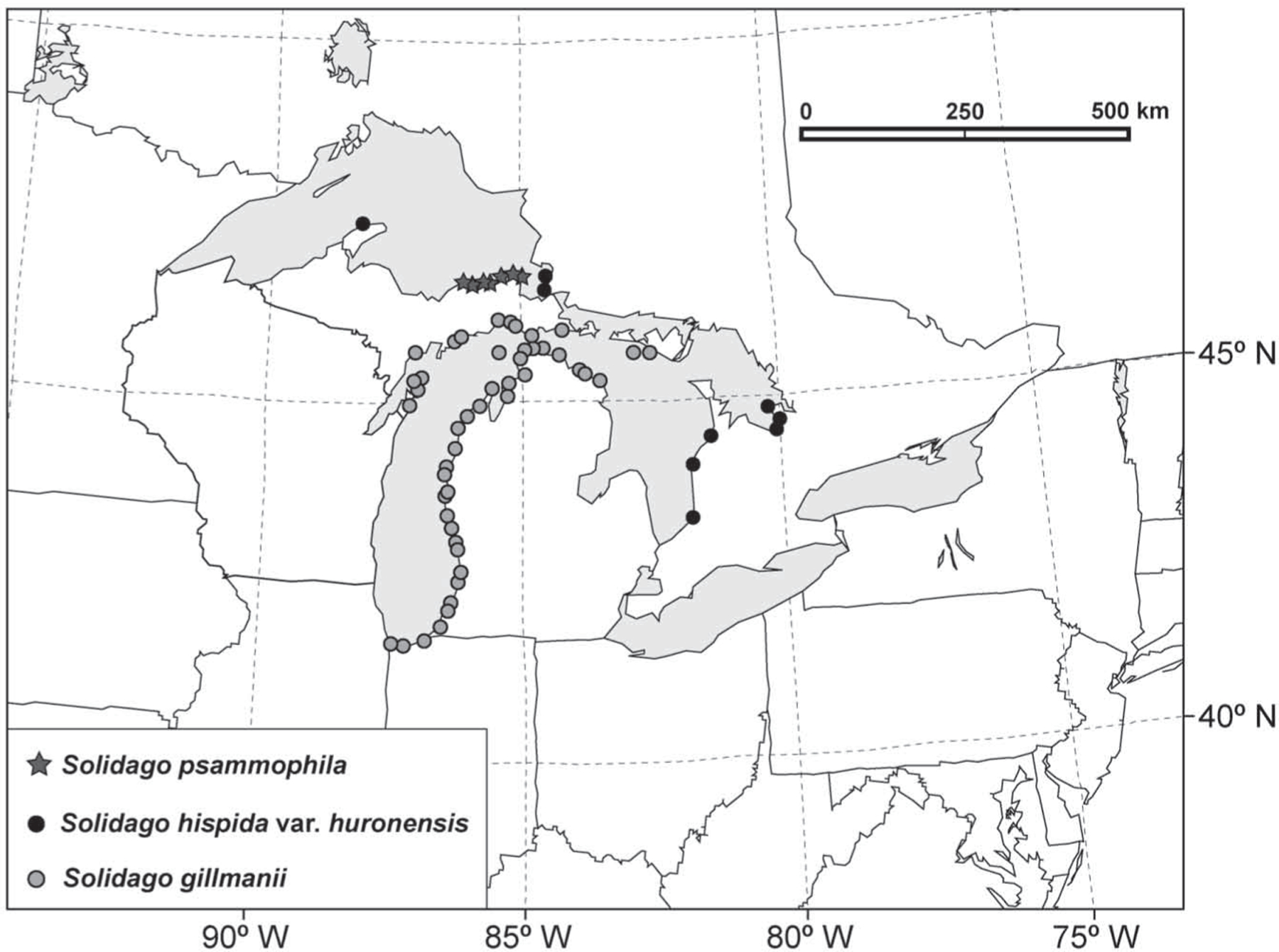
Solidago psammophila is restricted to active sand dunes along the southern shore of Lake Superior in Alger, Chippewa, and Luce Counties, Michigan (Figures 3 and 4). Populations of the species occur along an approximately 100 km length of shoreline from Grand Sable Dunes in the west to Whitefish Point in the east. No additional populations have been located along the Lake Superior shoreline west of Grand Sable Dunes. Solidago psammophila is a component of the open dune community (following Kost et al. 2007) and is commonly associated with other open dune species such as Ammophila breviligulata Fern., Artemisia campestris L., and Arctostaphylos uva-ursi (L.) Spreng. It is less frequently found at the periphery of more stabilized areas like Great Lakes barrens and bluffs adjacent to open dune habitats.
Distribution of sand dune endemic goldenrods in the North American Great Lakes region: Solidago gillmanii (gray circles), S. hispida var. huronensis (black circles), and S. psammophila (gray stars). Distributions based on the current study, Semple et al. (1999), and Peirson (2010).
Adaptation to the Dune Environment.
The sand dune environment exerts strong selection on plants that grow there (e.g., through factors like nutrient deficiency, drought, and recurrent sand burial), and sand dune specialists have developed adaptations to survive the extreme environment (Maun 1994, 1998). Solidago psammophila produces elongate vertical rhizomes that appear to be apparent adaptations to help survive sand burial. Greenhouse germinated and grown seedlings of S. psammophila, as well as those of S. gillmanii, produce elongate vertical stems below the rosette of leaves during the first year of growth, without any exposure to burial by sand. Contrastingly, greenhouse germinated and grown seedlings of the widespread S. glutinosa Nutt. from northern Michigan sand barren sites (where there is little or no sand movement) do not produce elongate vertical stems. Field observations from dune systems throughout the Great Lakes region have shown that some widespread species of Solidago commonly occur at the margins of the open dune habitat where the sand has become stabilized. These species, which presumably lack specific adaptations, are essentially absent from the inhospitable open dune environment.
DNA Ploidy.
Data from flow cytometry showed Solidago psammophila is uniformly tetraploid (4x = 36) across its range (Table 2).
Additional Specimens Examined.
U.S.A., MICHIGAN: S. shore of Lake Superior, 1875, W. Boott s.n. (GH); ALGER COUNTY: Grand Sable Dunes west of Grand Marais, T49N R14W sec. 10, 24 Sept 1964, R.C. Harris s.n. (MSC); Grand Sable Dunes, on the open dunes N of Grand Sable Lake, 5 Sept 1999, M. Chamberland 1306 (MSC); ca. 2.5 mi W of Grand Marais, common on sand dunes just N of Grand Sable Lake, 10 Aug 1954, E.G. Voss 2477 (MICH); open sandy area on Grand Sable Dunes, N of Grand Sable Lake, 3 Aug 1975, D. Bach 22 (MICH); CHIPPEWA COUNTY: west of Whitefish Point, plants common on stabilized and active sand dunes, 21 Aug 2010, J. A. Peirson 857 (MICH); Vermillion, along Lake Superior, upper beach and dunes, 9 Sept 1951, H.H. Bartlett & C.D. Richards 320 (MICH); near Vermillion, sandy beach of Lake Superior shore, 31 Aug 1914, C.K. Dodge s.n. (MICH); Whitefish Point, growing on shingle beach, 1 Aug 1977, W.T. Gillis 14073 (MSC—2 sheets); Whitefish Point, growing on shingle beach, 1 Aug 1977, W.T. Gillis 14074 (MSC); Whitefish Point, near Lake Superior, sparsely wooded dune-marsh area, on open dune, 9 Aug 1948, R. McVaugh 9768 (MICH, UMBS); LUCE COUNTY: ca. 1 mile east of Deer Park, active sand dunes on lake Superior, 24 Aug 2006, J. A. Peirson 638 (MICH); ca. 1 mile E of Deer Park, on sand dunes along Lake Superior shore, active sand dunes and somewhat stabilized sand toward base of bluff, 11 Sept. 2010, J.A. Peirson 861 (MICH); ca. 3–4 miles E of Deer Park, plants common on dunes along lake Superior, 25 Aug 2006, J. A. Peirson 641 (MICH); mouth of Three-mile Creek east along Lake Superior shore to Crisp Point Lighthouse, off of Luce County 412, plants common on active sand dunes and in dry, stabilized, interdunal meadows, 5 Sept 2008, J. A. Peirson 833 (MICH); east of the mouth of Three-mile Creek along Lake Superior shore, plants common on active sand dunes, 22 Aug 2021, J. A. Peirson 923 (MICH); Lake Superior Campground Beach, off of County Road 407 between Grand Marais and Deer Park, plants common along road and on stabilized sand bluff and more active dunes, 21 Aug 2010, J. A. Peirson 855 (MICH); near E edge of Sect. 3, T49N R10W, ca. 4 miles E of Deer Park, frequent, low dune ridge above Lake Superior, 21 Aug 1978, E.G. Voss 15011 (MICH); County Rd. 407, at mouth of Blind Sucker River, in coastal sand dunes, 27 Aug 1993, H.H. Schmidt & M. Merello 1074 (MO, MIN (online image), TEX).
BRIEF NOTES ON ENDEMIC SOLIDAGO TAXA IN THE GREAT LAKES REGION
In addition to Solidago psammophila and S. gillmanii, four other goldenrods have commonly been recognized as endemic to the glaciated North American Great Lakes region. Except for S. hispida var. huronensis Semple, which is diploid, all recognized Great Lakes endemic taxa of Solidago are polyploid (Table 3). Like the majority of the broader endemic flora, these Solidago taxa have distributions centered in the northern parts of the region. They are restricted to regionally rare, non-forested habitats that are linked to present and/or past Great Lakes shorelines (Peirson 2010).
Distribution, habitat, and ploidy of endemic taxa of Solidago in the glaciated North American Great Lakes region. Data summarized from Laureto and Pringle (2010), Peirson et al. (2012), Semple et al. (1999), Semple and Cook (2006), Voss and Reznicek (2012), and the current study.
| Taxon | Ploidy | Distribution | Habitats |
|---|---|---|---|
| Solidago gillmanii | 4x = 36 | Lakes Huron and Michigan shoreline | Open dunes |
| Solidago hispida var. huronensis | 2x = 18 | Lakes Huron and Superior shoreline | Open dunes |
| Solidago houghtonii | 6x = 54 | Lakes Huron and Michigan shoreline | Interdunal swales, sandy shores, alvars |
| Solidago ontarioensis | 4x = 36 | Lakes Huron, Michigan*, and Superior shoreline | Rock outcrops |
| Solidago psammophila | 4x = 36 | Lake Superior shoreline | Open dunes |
| Solidago vossii | 8x = 72 | Northern Lower Peninsula of Michigan (former postglacial shoreline) | Moist, sandy swales (inland) |
*A population of Solidago ontarioensis on limestone bedrock at Seul Choix Point, Schoolcraft County, Michigan, is the only occurrence of the species along the Lake Michigan shoreline.
Solidago hispida var. huronensis.
This taxon, along with Solidago psammophila and S. gillmanii, constitute the three sand dune endemic goldenrods that occur in the Great Lakes region. Solidago hispida var. huronensis is most common on active dune systems along the Canadian shores of Lake Huron, including Georgian Bay, but it also occurs along the shores of Lake Superior (both along the southeastern shore in Canada and on Michigan’s Keweenaw Peninsula in western Lake Superior). Whereas S. psammophila and S. gillmanii are cohesive, morphologically well-defined tetraploid species, diploid S. hispida var. huronensis appears to intergrade with more typical S. hispida along the open-dune to stabilized-dune transition at some locations. Glabrous plants that are most common on sparsely vegetated open dunes tend to be replaced by sparsely pubescent plants along the back-dune, which are in turn replaced by typical S. hispida individuals where the dunes become stabilized (J. Peirson, personal observations from Great Sand Bay, Keweenaw County, Michigan and Pinery Provincial Park, Ontario). The evolution of this Great Lakes endemic has not been closely studied, but Semple et al. (2017b) proposed that it likely represents an ecotype adapted to local conditions (possibly including sandy habitats further east in Ontario). Given its scattered, disjunct distribution, it seems plausible that this form may have evolved multiple times in response to site-specific edaphic conditions.
Solidago houghtonii.
Douglas Houghton first collected the flat-topped, large-headed Solidago houghtonii along the shores of northern Lake Michigan in Mackinaw Co., Michigan, on August 15, 1839 (Voss 1978). One of the best-known Great Lakes endemics, this hexaploid goldenrod is restricted to sandy and rocky shores and interdunal hollows of northern Lakes Michigan and Huron (Guire and Voss 1963; Morton 1979; Morton and Venn 2000; Laureto and Barkman 2011). A disjunct population in the Bergen Swamp, Genesee Co., New York has often been included in S. houghtonii (Guire and Voss 1963; Semple and Cook 2006; Laureto and Barkman 2011); however, its relationship to populations within the main Great Lakes distribution has not been definitively studied. Morton (1979) proposed that hexaploid S. houghtonii is an allopolyploid derivative of a cross between S. ohioensis Riddell (2n = 18) and S. ptarmicoides (Torr. & A. Gray) Boivin (2n = 18) with a subsequent backcross to S. ohioensis, whereas Semple et al. (1999) proposed that S. riddellii Frank may be involved in its origin. Laureto and Barkman (2011) suggested, based on chloroplast DNA sequence data, that S. gigantea Ait. was the maternal genome donor. Extensive cpDNA haplotype sharing within Solidago, however, has posed challenges for elucidating relationships within the genus more generally (e.g., Peirson et al. 2013).
Solidago vossii.
Inland populations of a flat-topped, large-headed goldenrod in northern Michigan (Crawford County) that had historically been included in Solidago houghtonii were recently described as S. vossii J.S. Pringle & Laureto (Laureto and Pringle 2010). The octoploid S. vossii is more robust and has larger involucres and ray florets than S. houghtonii. While its present distribution is inland, the locale lies along the shores of postglacial Lake Margrethe. The species occurs in a distinct wet sand prairie habitat that includes a mixture of plants common to mesic prairies as well as some species characteristic of Great Lakes interdunal wetlands (Laureto and Pringle 2010). Like S. psammophila, S. vossii is endemic to the state of Michigan.
Solidago ontarioensis.
This tetraploid endemic, recognized at the species level by Semple and Peirson (2013), formerly S. simplex var. ontarioensis (Ringius) Ringius, is restricted to shoreline rock outcrop habitats in the northern Great Lakes region. Plants grown in a common garden suggest that Solidago ontarioensis (Ringius) Semple & Peirson comprises two phenotypically distinct sets of populations in the Great Lakes region (Peirson 2010). Large-statured plants occur on dolomite shores of northern Lake Huron and northern Lake Michigan, along the boundary of the Niagara Escarpment. Smaller-statured plants occur primarily on granite/basalt outcrops along the southern and eastern shores of Lake Superior. This distribution of phenotypes raises the possibility that S. ontarioensis encompasses two independently derived lineages. Phylogeographic data suggest that the two groups have separate origins (Peirson 2010; Peirson et al. 2013); however, extensive haplotype sharing has thus far precluded any concrete assessment of evolutionary relationships.
KEY TO THE SPECIES OF SOLIDAGO OCCURRING IN MICHIGAN, U.S.A.
(modified with permission from Voss and Reznicek 2012)
-
1.
Heads in a terminal ± flat-topped corymbiform inflorescence.
-
2.
Blades of middle and upper cauline leaves ovate to elliptic (less than 3 times as long as broad), densely pubescent on both surfaces........................................................................................S. rigida
-
2.
Blades of middle and upper cauline leaves linear to lanceolate or oblanceolate (over 10 times as long as broad), glabrous or nearly so.
-
3.
Rays 12–18, white, 4.5–8 mm long; pappus hairs slightly but clearly thickened (slenderly clavate) toward tip; upper cauline leaves slightly oblanceolate (broadest above the middle)........................................................................................S. ptarmicoides
-
3.
Rays 10 or fewer, yellow, not over 4.5 (−7) mm long; pappus hairs not thickened (or some thickening scarcely visible in S. houghtonii); upper cauline leaves broadest at or below the middle.
-
4.
Rays 1.5–3 mm long and involucre ca. 3.5–5.5 (−6.5) mm long; pedicels smooth and glabrous or rough-hispidulous.
-
5.
Pedicels smooth and glabrous or nearly so; leaf blades with one longitudinal vein (but often some principal lateral veins), flat........................................................................................S. ohioensis
-
5.
Pedicels and inflorescence branches densely rough-hispidulous; leaf blades with 3 or more longitudinal veins at the base, all or mostly complicate........................................................................................S. riddellii
-
5.
-
4.
Rays 3–4.5 (−7) mm long and involucre ca. 5–9 mm long; pedicels scabrous-hispidulous.
-
6.
Larger involucres 5–7 (−8) mm long; larger plants mostly 30–60 cm tall; basal leaves entire; hexaploid; plants occurring on or near the Great Lakes shores, centered on the Straits of Mackinac........................................................................................S. houghtonii
-
6.
Larger involucres 7–9 mm long; larger plants mostly 50–80 cm tall; basal leaves sparsely serrulate; octoploid; inland in swales among Pinus banksiana........................................................................................S. vossii
-
6.
-
4.
-
3.
-
2.
-
1.
Heads in an elongate or pyramidal inflorescence or in axillary clusters.
-
7.
Inflorescence terminal, often ± pyramidal (broadest toward base, about equally long, slightly nodding at top) but sometimes grading into axillary branches, and with curving, one-sided branches (the heads mostly directed upwards on well-developed branches).
-
8.
Cauline leaves (at least the main ones) “triple-nerved,” i.e., with a pair of elongate veins arising below the middle of the midrib and distinctly stronger than other lateral veins.
-
9.
Leaves entire, succulent; saline habitats (e.g., edges of heavily salted roads)........................................................................................S. sempervirens
-
9.
Leaves with at least tiny and/or irregular teeth, of normal herbaceous texture; various habitats.
-
10.
Axis, pedicels, and branches of inflorescence glabrous; prairie and dry prairie-like habitats, blooming late in the season; lower and rosette leaves linear-lanceolate...................................................................S. missouriensis
-
10.
Axis, pedicels, and branches of inflorescence at least sparsely but distinctly pubescent; or if glabrous (S. juncea), the lower and rosette leaves much larger than the mid-cauline leaves, ± elliptic, and the plant blooming early in the season in dry habitats.
-
11.
Stem glabrous all of its length below the inflorescence, rarely with a few scattered, spreading, short hairs.
-
12.
Basal leaves none; cauline leaves narrowly (rarely broadly) elliptic and the lowest withered by flowering time; middle and upper cauline leaves crowded (numerous), about the same size as the lowest leaves or larger, and distinctly 3-nerved; plants blooming late (starting August–September); branches of inflorescence ± densely pubescent........................................................................................S. gigantea
-
12.
Basal (including rosette) and lower cauline leaves with oblanceolate to elliptic blades and long petioles, persistent; middle and upper cauline leaves remote (relatively few), distinctly smaller than basal leaves, and only weakly 3-nerved; plants blooming early (starting in July); branches of inflorescence glabrous or occasionally sparsely spreading pubescent..........................................................S. juncea (in part)
-
12.
-
11.
Stem pubescent all or most of its length.
-
13.
Involucres all or mostly 3.1–4.6 (−5) mm long........................................................................................S. altissima
-
13.
Involucres all or nearly all 2–3 mm long........................................................................................S. canadensis
-
13.
-
11.
-
9.
-
8.
Cauline leaves with distinct midrib but the other (weaker) veins ± pinnate.
-
14.
Stems ± pubescent, at least on the upper half of the plant.
-
15.
Cauline leaves entire or obscurely crenate-toothed; leaves and stems uniformly and densely puberulent throughout; lower and basal (including rosette) leaves oblanceolate, tapered into a winged petiole and larger than mid-cauline leaves; sandy or rocky, open and usually very dry soil......................................S. nemoralis
-
15.
Cauline leaves sharply toothed; leaves beneath (at least on main veins) and stem with mostly spreading, longer hairs (over 0.5 mm); lower and basal leaves (none in rosettes) no larger than mid-cauline leaves (but usually absent at flowering time), all of them elliptic-lanceolate; moist or shaded ground........................................................................................S. rugosa
-
15.
-
14.
Stems glabrous (except sometimes just below and in the inflorescence).
-
16.
Lowest cauline leaves with tapering base clasping stem (encircling it for at least half its circumference); wet habitats, with leaves nearly smooth above..........................................S. uliginosa (in part)
-
16.
Lowest cauline leaves not clasping stem; dry habitats or, if wet, the leaves very scabrous above.
-
17.
Stem with strongly raised angles or ribs; upper leaf surface very scabrous, with dense, tiny, stiff conical projections; swamps and other wet habitats..............................S. patula
-
17.
Stem terete (may be many-ridged); upper leaf surface smooth to slightly scabrous; ± dry open or forested habitats.
-
18.
Basal (including rosette) and lower cauline leaves much larger than mid-cauline leaves, persistent (blades often 7–20 cm long on petioles half or more as long); branches of inflorescence glabrous or occasionally sparsely spreading-pubescent; leaves often tending to have prominent longitudinal veins, usually glabrous beneath but occasionally with some hairs on midrib; throughout Michigan, beginning to bloom in July (before other goldenrods)................................................S. juncea (in part)
-
18.
Basal and lower leaves often withered by flowering time or, if present, not much larger than mid-cauline leaves; branches of inflorescence rather densely pubescent; leaves clearly pinnate-veined, with midrib and principal veins beneath spreading-pubescent (as in S. rugosa); southern Lower Peninsula, blooming late........................................................................................S. ulmifolia
-
18.
-
17.
-
16.
-
14.
-
8.
-
7.
Inflorescence axillary or terminal, but even if pyramidal the branches not one-sided and the top not nodding.
-
19.
Leaves decreasing in size from middle of stem to the base, the mid- to upper cauline leaves sharply toothed, much exceeding the distinctly axillary inflorescences (not necessarily any branches) they subtend; stems glabrous (except rarely on upper internodes), the lowest leaves usually withered by flowering time; achenes ± densely pubescent.
-
20.
Leaf blades narrowly elliptic, sessile; stem terete, glaucous when fresh, not (or scarcely) zigzag; leaves glabrous (except for short-ciliate margin); cespitose......................................S. caesia
-
20.
Leaf blades broadly ovate-elliptic, abruptly contracted to a winged petiole; stem ribbed or angled throughout, ± zigzag from node to node; leaves (at least the midrib beneath and petiole margins) ± sparsely pubescent; colonial from creeping rhizomes......................................S. flexicaulis
-
20.
-
19.
Leaves increasing in size from middle of stem to the base, the mid-cauline leaves usually entire to crenate-toothed and usually not subtending inflorescences (these more clearly terminal); stems glabrous or pubescent, the lowest leaves usually persistent; achenes glabrous or glabrate (except in S. psammophila and the S. glutinosa group).
-
21.
Stem sparsely to densely pubescent its entire length and leaves pubescent, at least abaxially.
-
22.
Rays white or cream when fresh.................................................S. bicolor
-
22.
Rays yellow
-
23.
Stems usually numerous (5–8), from deep vertical rhizomes; involucres 5–8 mm long; achenes antrorse-strigose; tetraploid; active coastal sand dunes of Lake Superior...............................S. psammophila
-
23.
Stems solitary or few (1–3), from shallow caudices; involucres 3–4.5 mm long; achenes glabrous or glabrate; diploid; usually dry habitats, including margins of coastal sand dunes.......................................S. hispida (in part)
-
23.
-
22.
-
21.
Stem glabrous at least below the middle and leaves generally glabrous.
-
24.
Lower cauline leaves ca. 6–18 times as long as broad, the petiole clasping the stem for half or more of its circumference; plants occurring in wet habitats (including rock crevices on Lake Superior).....................................................S. uliginosa (in part)
-
24.
Lower cauline leaves ca. 3–8 times as long as broad, not clasping (leaves of basal rosettes sometimes as much as 11 times as long as broad); plants occurring mostly in dry habitats.
-
25
Achenes antrorse-strigose; involucres and leaves resinous (more easily determined when fresh, but usually appearing varnished, shiny or glandular when dry).
-
26.
Plants robust; stems from deep vertical rhizomes; petiole ciliate; plants flowering mid August to October; active coastal sand dunes of Lakes Huron and Michigan........................................................................................S. gillmanii
-
26.
Plants relatively small; stems from shallow caudices; petiole usually not ciliate; plants flowering late June to mid August; coastal rock outcrops of Lakes Michigan and Superior or inland sand barrens.
-
27.
Involucres 4.5–6.2 mm long; plants flowering mid July to mid August; tetraploid; coastal rock outcrops of the Upper Peninsula ........................................................................................S. ontarioensis
-
27.
Involucres 3.4–4.3 mm long; diploid; plants flowering late June to late July; inland sand barrens of the Lower Peninsula............S. glutinosa
-
27.
-
26.
-
25
-
25.
Achenes glabrous or glabrate; involucres and leaves not resinous.
-
28.
Cauline leaves (3–) 5–15 (–17) below inflorescence; margins of lower and middle leaves crenulate; plants occurring on rock outcrops and dunes on Lake Superior and northern lake Huron.........S. hispida (in part)
-
28.
Cauline leaves ca. (11–) 15–30 below inflorescence; margins of lower and middle leaves entire to sparsely toothed in the upper half; plants occurring in prairies, jack pine plains, and oak barrens, sandy fields and rock outcrops (inland).
-
29.
Basal rosette and lower stem leaves present at flowering; plants flowering from early July to late August; jack pine plains, sandy fields, and rock outcrops of the northern Lower Peninsula and western Upper Peninsula.............................................................................S. jejunifolia
-
29.
Basal rosette and lower stem leaves absent at flowering; plants flowering September and October; prairies, sandy fields, and oak barrens of the southern half of the Lower Peninsula.............S. rigidiuscula
-
29.
-
28.
-
24.
-
21.
-
19.
-
7.
ACKNOWLEDGMENTS
I am grateful to the curators and staff at GH, MICH, MO, MSC, MT, TEX, and UMBS for their assistance and for the loan of or access to specimens. I also wish to thank the late Ed Voss (MICH) for introducing me to the dunes and goldenrods along the southern shore of Lake Superior; Tony Reznicek (MICH) for permitting me to adapt the Solidago key from Field Manual of Michigan Flora, for many discussions about the Great Lakes flora, and for providing critical comments on earlier versions of the manuscript; Andy Larson for assistance in the field and greenhouse; and Paul Kron and Brian Husband (University of Guelph, Canada) for their expertise in performing the flow cytometry. Funding for this project was provided by the Hanes Fund.
LITERATURE CITED
Doležel, J., P. Binarová, and S. Lucretti. (1989). Analysis of nuclear DNA in plant cells by flow cytometry. Biologia Plantarum 31: 113–120.
Doležel, J., M. Doleželová, and F. J. Novák. (1994). Flow cytometric estimation of nuclear DNA amount in diploid bananas (Musa acuminata and M. balbisiana). Biologia Plantarum 36: 351–357.
Doležel, J., J. Greilhuber, and J. Suda. (2007). Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols 2: 2233–2244.
Dorr, J. A., and D. F. Eschman. (1970). Geology of Michigan. University of Michigan Press, Ann Arbor.
Gray, A. (1882). Contributions to North American botany. Proceedings of the American Academy of Arts and Sciences 17: 163–230.
Gross, R. S., and P. A. Werner. (1983). Relationships among flowering phenology, insect visitors, and seed-set of individuals: Experimental studies on four co-occurring species of goldenrod (Solidago: Compositae). Ecological Monographs 53: 95–117.
Guire, K. E., and E. G. Voss. (1963). Distributions of distinctive shoreline plants in the Great Lakes region. The Michigan Botanist 2: 99–114.
Halverson, K., S. B. Heard, J. D. Nason, and J. O. Stireman. (2008). Origins, distribution, and local co-occurrence of polyploid cytotypes in Solidago altissima (Asteraceae). American Journal of Botany 95: 50–58.
Havercamp, J., and G. G. Whitney. (1983). The life history characteristics of three ecologically distinct groups of forbs associated with the tallgrass prairie. The American Midland Naturalist 109: 105–119.
Hiddeman, W., J. Schumann, M. Andreef, B. Barlogie, C. J. Herman, R. C. Leif, B. H. Mayall, (1984). Convention on nomenclature for DNA cytometry. Cytometry 5: 445–446.
Hood, J. L. A., and J. C. Semple. (2003). Pappus variation in Solidago (Asteraceae: Astereae). Sida 20: 1617–1630.
Kost, M. A., D. A. Albert, J. G. Cohen, B. S. Slaughter, R. K. Schillo, C. R. Weber, and K. A. Chapman. (2007). Natural communities of Michigan: Classification and description 2007–21, Michigan Natural Features Inventory, Lansing.
Laureto, P. J., and T. J. Barkman. (2011). Nuclear and chloroplast DNA suggest a complex single origin for the threatened allopolyploid Solidago houghtonii (Asteraceae) involving reticulate evolution and introgression. Systematic Botany 36: 209–226.
Laureto, P. J., and J. S. Pringle. (2010). Solidago vossii (Asteraceae), a new species of goldenrod from northern Michigan. The Michigan Botanist 49: 105–117.
Maun, M. A. (1994). Adaptations enhancing survival and establishment of seedlings on coastal dune systems. Vegetatio 111: 59–70.
Maun, M. A. (1998). Adaptations of plants to burial in coastal sand dunes. Canadian Journal of Botany 76: 713–738.
Morton, J. K. (1979). Observations on Houghton’s goldenrod (Solidago houghtonii). The Michigan Botanist 18: 31–35.
Morton, J. K., and J. M. Venn. (2000). The flora of Manitoulin Island, third edition. Department of Biology, University of Waterloo, Waterloo, Ontario.
Peirson, J. A. (2010). Biogeography, ecology, and evolution of the endemic vascular flora of the glaciated Great Lakes region: A case study of the Solidago simplex species complex. PhD Thesis, The University of Michigan, Ann Arbor.
Peirson, J. A., C. W. Dick, and A. A. Reznicek. (2013). Phylogeography and polyploid evolution of North American goldenrods (Solidago subsect. Humiles, Asteraceae). Journal of Biogeography 40: 1887–1898.
Peirson, J. A., A. A. Reznicek, and J. C. Semple. (2012). Polyploidy, infraspecific cytotype variation, and speciation in goldenrods: the cytogeography of Solidago subsect. Humiles (Asteraceae) in North America. Taxon 61: 197–210.
Ringius, G. S. (1987). Lectotypifications and a new combination in the Solidago spathulata DC. –S. glutinosa Nutt. complex (Compositae: Astereae). Taxon 36: 154–157.
Schlaepfer, D. R., P. J. Edwards, J. C. Semple, and R. Billeter. (2008). Cytogeography of Solidago gigantea (Asteraceae) and its invasive ploidy level. Journal of Biogeography 35: 2119–2127.
Semple, J. C. (2022). Classification and illustrations of goldenrods. Available at https://uwaterloo.ca/astereae-lab/research/goldenrods/classification-and-illustrations.https://uwaterloo.ca/astereae-lab/research/goldenrods/classification-and-illustrations (Accessed October 9, 2022).
Semple, J. C., and J. B. Beck. (2021). Revised infrageneric classification of Solidago (Asteraceae: Astereae). Phytoneuron 2021- 10: 1–6.
Semple, J. C., and R. E. Cook. (2006). Solidago. Pp. 107–166 in Flora of North America, volume 20: Magnoliophyta: Asteridae (in part): Asteraceae, Part 2. Flora of North America Editorial Committee, editors. Oxford University Press, New York, N.Y.
Semple, J. C., H. McMinn-Sauder, M. Stover, A. Lemmon, E. Lemmon, and J. B. Beck. (2022, submitted). Goldenrod herbariomics: Hybrid sequence capture reveals the phylogeny of diploid Solidago. American Journal of Botany.
Semple, J. C., and J. A. Peirson. (2013). Revised nomenclature for the Solidago simplex complex (Asteraceae: Astereae). Phytoneuron 2013: 1–5.
Semple, J. C., G. S. Ringius, and J. J. Zhang. (1999). The goldenrods of Ontario: Solidago L. and Euthamia Nutt., third edition. University of Waterloo Biology Series 39: 1–90.
Semple, J. C., L. Tong, and Y. A. Chong. (2017a). Multivariate studies of Solidago subsect. Squarrosae. I. The Solidago speciosa complex (Asteraceae: Astereae). Phytoneuron 2017- 18: 1–23.
Semple, J. C., L. Tong, Y. A. Chong, and M. Kaddoura. (2017b). Multivariate studies of Solidago subsect. Squarrosae. II. The Solidago bicolor –S. hispida complex (Asteraceae: Astereae). Phytoneuron 2017- 33: 1–44.
Trick, J. A., and G. Fewless. (1984). A new station for Dwarf Lake Iris (Iris lacustris) in Wisconsin. The Michigan Botanist 23: 68.
Voss, E. G. (1978). Botanical beachcombers and explorers: Pioneers of the 19th century in the upper Great Lakes. Contributions from the University of Michigan Herbarium 13: 1–100.
Voss, E. G. (1996). Michigan Flora, Part III: Dicots (Pyrolaceae–Compositae). Cranbrook Institute of Science, Bulletin 61, Bloomfield Hills, Michigan.
Voss, E. G., and A. A. Reznicek. (2012). Field manual of Michigan flora. The University of Michigan Press, Ann Arbor.
Williams, M., D. Dunkerley, P. De Deckker, P. Kershaw, and J. Chappell. (1998). Quaternary environments. second edition. Arnold, London.