Introduction
Plaster Creek in Kent County, Michigan, with its remnant floodplain habitats presents a unique case-study for how drastically urban, suburban, and agricultural development have altered the native biodiversity and ecological integrity of the stream and its watershed over time. Plaster Creek, whose headwaters are located southeast of Grand Rapids in the agricultural areas of Dutton and Caledonia (FTC&H 2008), drains a watershed of approximately 58 square miles. From these headwaters the creek meanders through the present-day City of Kentwood and into the southern portion of the City of Grand Rapids, flowing through residential, commercial, and industrial areas, before joining the Grand River about one mile south of the city center.
Plaster Creek’s watershed has undergone extensive changes since the field work of Emma Cole in the 1890s. Cole, a local botanist and science educator, visited a variety of locations along Plaster Creek, which she highlighted in her Grand Rapids Flora (Cole 1901). Although larger expanses of natural habitat existed along Plaster Creek at the time of Cole’s botanical work in the 1890s compared to today, the creek and its corridor had already been subjected to significant disturbances in her day. In 1837 Douglass Houghton (1839), while conducting the first geological survey of Michigan, visited West Michigan to investigate salt springs for possible mining. Although his search for salt was only somewhat productive, he was especially impressed by high quality gypsum out-crops (Houghton 1838) along the Ken-O-Sha (Kee-No-Shay), the original Ottawa name for Plaster Creek, which means “Water of the Walleye” (Belknap 1922, 1926).
Only three years later, in 1841, the first gypsum mine in Grand Rapids was established by Warren Granger and Daniel Ball at the site where Plaster Creek formed a waterfall that flowed over a gypsum ledge into the Grand River floodplain (Grimsley 1904). This location was known to be a sacred site for the Ottawa people, but in a few short years it was obliterated by the mining operation. Once mined, the high-quality ground gypsum provided two marketable products: an agricultural fertilizer in powdered form, and a building material when mixed with water. By 1850, this mine was yielding 60 tons of gypsum daily, and the jobs and wealth it generated earned the area the title “Happy Valley.” However, the ecological fallout of this operation included clearing a large forested area, draining a wetland, and completely re-routing and channelizing the stream. In addition, the availability of local gypsum, also referred to as “land plaster,” advanced farming activity in the Grand Rapids area, which prompted additional forest loss. Tailings from the mining operation so polluted the stream that walleye stopped swimming up the creek to spawn, and sadly but fittingly the original name for the waterway, “Ken-O-Sha,” was replaced by “Plaster Creek” (Belknap 1922).
Forest clearing began in West Michigan in the late 1700s and had profound impacts on Plaster Creek. Trees were first logged for homesteading and for agriculture and later for income generation. As forest cover declined along the upper reaches of Plaster Creek, the creek’s flow cycles intensified. In 1910, Charles Garfield, who grew up in the Plaster Creek Watershed and became the first president of Michigan’s Forestry Commission, wrote about the impacts of deforestation on the creek (Garfield 1910):
[Plaster Creek] has almost nothing now in the way of tree growth from its source to its confluence with the Grand River, and instead of being the beautiful even-flowing stream throughout the year, as in my childhood, it is now a most fitful affair, full to the brim and running over at times, yet most of the year it is only a trickling rill ….
The extreme flow cycles that Garfield (1910) lamented have further intensified today, now exacerbated by the replacement of native vegetation with expansive impermeable surfaces (roads, parking lots, rooftops, etc.) throughout Plaster Creek’s watershed. These changes to the landscape feed excessive amounts of stormwater runoff directly into the creek during rain events. The erosive forces caused by extreme runoff volumes result in high sediment loads, toxic levels of E. coli, and countless other harmful substances, such as road salts, pesticides, fertilizers, and hydrocarbons collecting in the creek, all of which have been documented by Calvin University researchers. By the early 2000s, as these pollutants proliferated in the creek, the Michigan Department of Environmental Quality labeled Plaster Creek as the most contaminated waterway in West Michigan, due in large part to the ecologically uninformed ways that development occurred in the watershed over time (Lee and Warners 2014; DeJong 2017).
One of the ways that uninformed development ecologically damaged Plaster Creek is by destroying much of its floodplain. Although a handful of intact, functional floodplain remnants do still exist along Plaster Creek (such as the parcels we inventoried for this project), most of the creek’s original floodplain has been lost to residential neighborhoods, commercial properties, and industrial zones. The importance of the remaining healthy, intact floodplain habitat within the Plaster Creek watershed cannot be overstated. Into these floodplain zones the creek overflows during periods of high volume, and floodplain vegetation slows the water, allowing water-borne sediment to fall out of stream flow. In addition, water percolates into the floodplain soils, where plants transpire large volumes of it into the air (Hopkins 1999). These features of healthy floodplains mean that they not only reduce flooding frequency and intensity but also cause cleaner water to be transported downstream to lower reaches of Plaster Creek, the Grand River, and eventually, Lake Michigan. Furthermore, floodplains support a rich assemblage of native Michigan plants that in turn support a broad array of insects, birds, and mammals. In these ways, healthy floodplain ecosystems support healthy ecological and human communities in the Plaster Creek watershed.
Overview of Floristic Quality Assessments
History and Development
Floristic Quality Assessments provide useful metric-based measures to evaluate habitat conservation value and have become increasingly influential in North America over the past 20 years (Spyreas 2019). Conservation practitioners and land managers often have a fundamental need to be able to rapidly assess the value of various land parcels with respect to natural quality and ecological integrity and thus conservation value. Well-seasoned field biologists can often make an initial professional assessment to suggest which lands may be of higher priority for preservation or restoration, but this type of evaluation involves subjective judgments. Methods that yield objective and quantitative ecological indicators are preferable to standardize and guide such assessments. However, care must be taken when using simple assessments, which may provide little information about complex vegetation properties, such as ecological uniqueness, floristic composition, influence of non-native species, and regional distinctiveness (Spyreas 2019).
It was with these considerations in mind that the authors of Plants of the Chicago Region developed their objective metrics for rating the natural quality of plant communities (Swink and Wilhelm 1979). Originally referred to as the “Natural Areas Rating Index” Swink and Wilhelm (1994) later modified and refined their rating system, renaming the overall methodology Floristic Quality Assessment (FQA). The FQA system of Swink and Wilhelm (1994) is available for numerous states across the country. The Michigan FQA system was formulated in 2001 (Herman et al. 2001), and it includes detailed practical information on its application to Michigan natural areas.
Swink and Wilhelm (1994) recognized that certain species had a very high affinity for, or fidelity to, rather specific habitat conditions, whereas other plants could be found growing in a wide range of habitats. This led them to assign what they called a Coefficient of Conservatism value (C-value) to each native species, a value that was intended to reflect the level of fidelity each species had to its particular habitat. To illustrate this idea, one almost always encounters white fringed orchid (Platanthera blephariglottis) in pristine sphagnum bogs, and it is assigned a C-value of 10. In contrast, red maple (Acer rubrum) has a C-value of 1, since it may grow in a bog but can also be found in many wetland woods and can even be a major component of upland forests, particularly in northern areas.
Significance and Application
As a consequence of agricultural and urban development, logging, and hydrological alterations, many of the principal floristic elements of our presettlement ecosystems are poorly represented in Michigan’s present landscape (Herman et al. 2001). Much of Michigan’s remaining native biota has become severely restricted to small, isolated tracts of natural landscapes, which have themselves been impacted by surrounding growth and development (Zipperer 1993; Hartley and Hunter 1998; Warners et al. 2021; Crow et al. 2022). As a result, even small sites that house remnants of Michigan’s native biodiversity hold much significance, and objective quantitative tools such as FQA can be used to evaluate their conservation value.
Herman et al. (2001) have set FQA thresholds (Table 1), suggesting that sites with FQI scores of 35 or higher have floristically important statewide value. FQI scores greater than 50 suggest exceptional sites that exhibit extremely high conservation value and represent a significant component of Michigan’s native biodiversity and natural landscapes. Some feel that although a site’s FQI values are useful, a site’s mean C-value represents a less biased indicator of its relative conservation value, especially when comparing similar natural communities such as river floodplains (Matthews et al. 2005; Slaughter et al. 2015). However, Matthews et al. (2015) found that species co-occurred with others of similar C-value far more than expected by chance, thus affirming the reliability of FQAs. Slaughter et al. (2015) regard differences of mean C-values to be modest when calculated within a particular habitat type but to have significant differences if applied to sites that encompass a variety of habitat types. We consider both FQI and mean C-values to be helpful for practitioners involved in ecological integrity assessments, so we provide both in this paper.
Significance of different ranges of Native FQI as calculated under the Michigan Floristic Quality Assessment System for evaluating individual natural habitats as reflecting Michigan’s native biodiversity and natural landscapes, based on Herman et al. (2001).
| Native FQI | Significance of habitat quality to Michigan | Value of site to Michigan |
|---|---|---|
| < 20 | Minimal indication of natural quality; reflects much human disturbance. | Low value. |
| 21–34 | Average quality. | Moderate value. |
| 35–50 | Sufficient conservatism and richness in native flora; high quality. | Floristically important statewide. |
| > 50 | Rare, highly specialized or extraordinarily high quality; significant component of Michigan’s remaining native biodiversity. | Extremely high value; worthy of protection and conservation. |
Materials and Methods
Descriptions of the Seven Remnant Floodplain Sites
Over the past decade, the Emma Cole Grand Rapids Flora Project inventoried seven remnant floodplain sites along Plaster Creek from near its source in rural Gaines Township to the crossing of Plaster Creek at Madison Avenue within the urban core of Grand Rapids (Figure 1). Much has changed since Cole’s day, as we have documented with the help of Cole’s (1901) detailed accounts and herbarium specimens. This paper reports the most thorough inventory of the remnant natural floodplain areas in the Plaster Creek corridor to date, providing valuable baseline reference data for the ambitious watershed restoration work that is being undertaken by Calvin University’s Plaster Creek Stewards (Calvin University 2023).The sites are listed in order from farthest upstream in Gaines Township to farthest downstream in the City of Grand Rapids.
Plaster Creek study sites: south to northwest (progressively downstream): Crystal Springs, Gaines Twp.; Paris Park, Kentwood; Wernlund Property, Kentwood; Covenant Park, Kentwood; Stanaback Park area, Kentwood; Ken-O-Sha Park, Grand Rapids; Madison Avenue Crossing, Grand Rapids. 1914 Topographic Map, Grand Rapids Quadrangle, Michigan Geologic Survey/USGS.
Crystal Springs (42° 50.758′N, 85° 35.602′W)
The Crystal Springs site of 2.1 ha is located in Gaines Township about one mile west of the center of the village of Dutton on the property of the Leisure Creek Condominium Association (Figure 2). Plaster Creek enters the parcel after crossing under 68th Street and flows northward through the condominium property in the northeastern portion of an area historically known as Crystal Springs. We know that Cole visited Plaster Creek at this location in the 1890s, even though she did not specifically mention Crystal Springs in her Flora of Grand Rapids (Cole 1901). Evidence that Cole collected here is confirmed by seven extant herbarium specimens she labeled as “Plaster Creek, Crystal Springs”—Carex emoryi, C. prairea, C. sterilis, C. tetanica, Euphorbia commutata, Hierchloe odorata, and Dichanthelium clandestinum—with field visits occurring May 19, 1894; May 10, 1896; May 19, 1897; and July 14, 1897 (MICHIGAN FLORA ONLINE 2011). Our inventory, conducted in 2018 at the invitation of the Leisure Creek Condominium Association and augmented in 2022, is documented by 73 voucher specimens.
Paris Park (42° 51.348′N, 85° 35.075′W)
Paris Park is an 18-ha site of undeveloped woodland along Plaster Creek that is owned by Kent County Parks (Figure 3A). There are several trails that are maintained by the City of Kentwood Parks and Recreation Department. Plaster Creek flows into the park under 60th Street SE from Gaines Township to the southwest, is joined by an unnamed tributary from the southeast, and then meanders northward towards 52nd Street SE. The park can be accessed from 60th Street SE just east of the intersection with Hanna Lake Avenue SE. Paris Park was inventoried in 2015 and 2016 by the Emma Cole Grand Rapids Flora Project and is documented by 143 voucher specimens.
Wernlund Family Property (42°51.784′N, 85° 35.049′W)
The Wernlund property floodplain is a small site of 2.3 ha on private land between East Paris Avenue and Wing Avenue in the City of Kentwood in a neighborhood accessed from the south side of 52nd Street (Figure 3B). Plaster Creek meanders through the property, entering from the south and meandering out to the north. The site is located immediately across the creek from Paris Park on the north side of Plaster Creek. The flora of the Wernlund site was inventoried in 2021 at the invitation of the property owners and is documented by 82 voucher specimens.
Covenant Park (42° 53.520′N, 85° 34.980′W)
Covenant Park occupies a large parcel of land (11.9 ha) at the southeast corner of the intersection of Shaffer Avenue and 36th Street within the City of Kentwood (Figure 4). Previously known as The Christian Reformed Recreation Center, which included Fellowship Greens Golf Course, the site is now under the ownership of the City of Kentwood and is maintained by their Parks and Recreation Department. The Plaster Creek corridor, along with its remnant forested floodplain sections, enters Covenant Park from the south, meanders through the property, and exits toward the northwest corner of the park under a bridge on Shaffer Avenue. After leaving Covenant Park, Plaster Creek eventually flows into the Stanaback Park floodplain area. While much of the creek’s natural floodplain in Covenant Park had been converted to fairways, some small floodplain forest remnants remain. These forested areas were the focus of our 2021 botanical inventory work, with 114 voucher specimens documenting its flora.
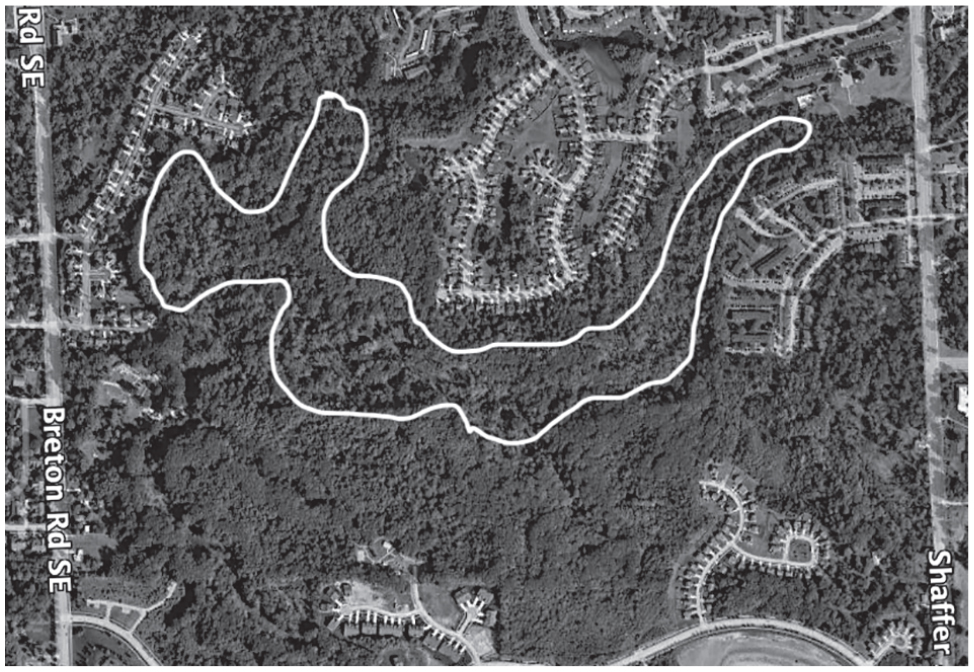
Stanaback Park Area (42° 53.800′N, 85° 35.945′W)
The Stanaback Park floodplain is 21.8 ha in size and represents the single largest remnant flood-plain inventoried by this project. It is located between Shaffer Avenue and Breton Road and bordered on the north by 32nd Street and by Pfeiffer Woods Drive on the south (Figure 5). The majority of this property is owned by the City of Kentwood, but some smaller privately owned parcels are included in the floodplain as well. The park features a large undeveloped wooded area adjacent to a small playground. The wooded area is comprised of a ravine system and the Plaster Creek floodplain. Plaster Creek enters the floodplain from Shaffer Avenue on the east and meanders through the site, exiting on the west under Breton Road. The floodplain has been protected by the ravine system that borders its southern edge and by the lack of recreational trails through the adjacent woods, which has minimized recent disturbance. Thus, this site represents a uniquely large intact remnant of Michigan’s native floodplain biodiversity in the mostly urbanized Plaster Creek watershed. The flora was inventoried in 2021 and is documented by 285 voucher specimens.
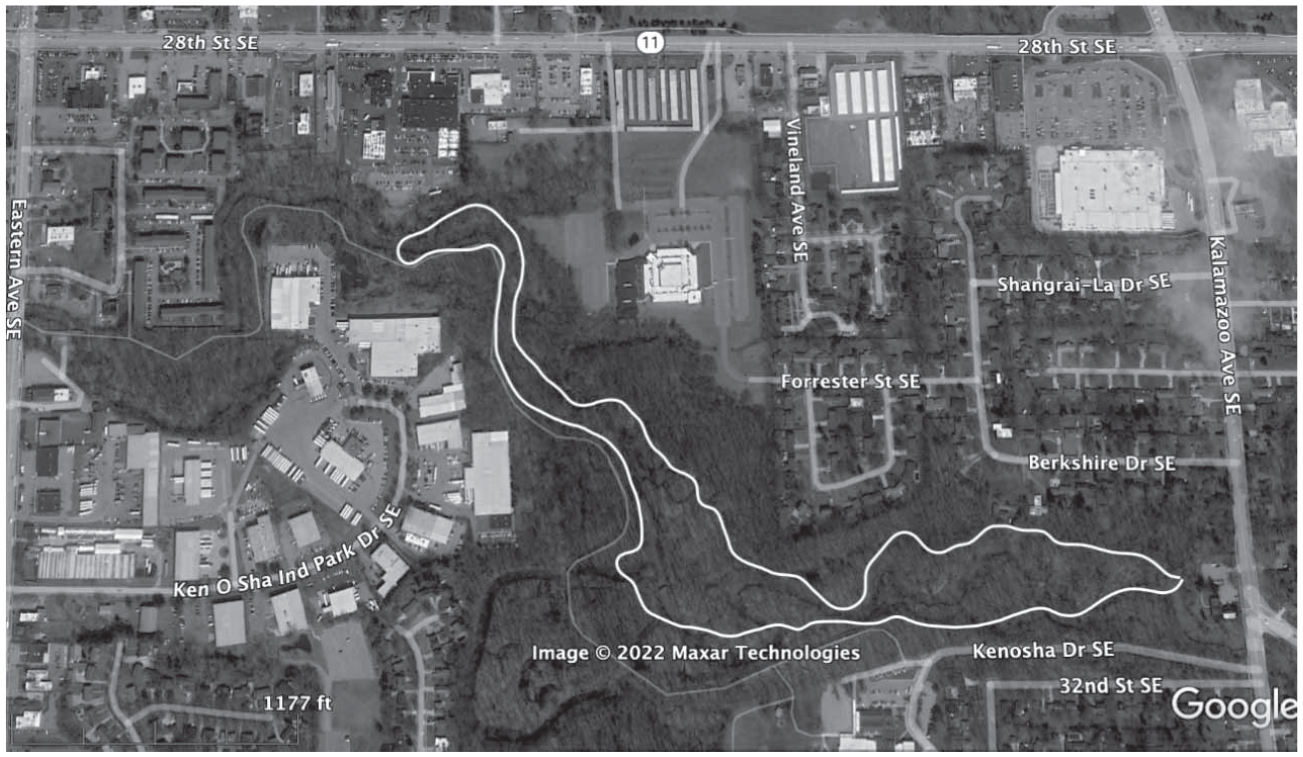
Ken-O-Sha Park (42° 54.397′N, 85° 38.165′W)
The Ken-O-Sha Park floodplain, at 9.5 ha, occurs within the City of Grand Rapids, straddling both sides of Plaster Creek’s main channel for nearly one mile just south of 28th Street (Figure 6). The park can be accessed on Ken-O-Sha Drive west of Kalamazoo Avenue. Plant collections contributing to the inventory of this site were made in 2012, 2015, and 2022 for the Emma Cole Grand Rapids Flora Project, and its flora is documented by 224 voucher specimens. This rich floodplain site is of particular interest to our project because Emma Cole (1901) references several plant species in her Grand Rapid Flora as occurring “near the Paris Town Hall,” a historic building that still stands on Kalamazoo Avenue adjacent to Plaster Creek and the entrance to Ken-O-Sha Park.
Plaster Creek Trail at Madison Avenue Crossing (42° 54.995′N, 85° 39.215′W)
The Plaster Creek Trail floodplain is a 5 ha site located within the City of Grand Rapids and can be accessed at the bridge on Madison Avenue near the intersection of Ken-O-Sha Drive (Figure 7). Plaster Creek Trail winds along the Plaster Creek channel here through a remnant of the creek’s floodplain. Four small, somewhat disconnected, parcels of floodplain vegetation are present along the north and south sides of the creek. Plaster Creek enters this site from the south at 28th Street (ca. 0.5 mi from the Ken-O-Sha Park site) between Eastern Avenue and Madison Avenue and then flows northwest under Madison Avenue from the west of the study site. The floodplain at this site bears significant evidence of disturbance, yet some large trees and other elements of natural floodplain diversity remain, especially on the less-accessible portion on the north side of the creek. We inventoried this site in 2021, and 122 voucher specimens document the floodplain flora. This floodplain is of particular interest to our project because Emma Cole documented several plant species at Madison Avenue along Plaster Creek, as noted in her Grand Rapids Flora (Cole 1901) and documented by Cole’s specimens on deposit at the University of Michigan Herbarium (MICHIGAN FLORA ONLINE 2011).
Botanical Inventory
During the growing seasons of 2012, 2015, 2016, 2018, 2021, and 2022, botanical inventories were conducted to assess sites along Plaster Creek, especially focusing on remnant floodplains. Sampling protocol for all sites was a meander-search throughout, conducted multiple times over the course of the growing seasons. All species encountered in the field were documented by voucher herbarium specimens or recorded as sight records. Identifications and nomenclature follow that of MICHIGAN FLORA ONLINE (2011), as this source includes both seed plants and pteridophytes and is periodically updated with taxonomic and nomenclatural changes. A total of 1,043 herbarium voucher specimens documenting the inventories are deposited in the Calvin University Herbarium (CALVIN); duplicates, where available, are deposited in the herbaria of Michigan State University (MSC) and/or University of Michigan (MICH).
In order to make comparisons between our inventories and the 1890s flora of Plaster Creek, Emma Cole’s (1901) Grand Rapids Flora was examined for species noted as occurring at “Plaster Creek.” Additionally, the MICHIGAN FLORA ONLINE (2011) database was also searched for specimens collected by Cole for which “Plaster Creek” appears on the label that might not have been so noted in her Flora. This yielded a list of 65 species that are likely to have occurred in the floodplain or in seepage areas at the base of steep ravines leading into the floodplain.
Floristic Quality Assessments
Floristic Quality Assessments (FQA) were conducted for each Plaster Creek floodplain site following the methodology described by Freyman et al. (2015) and Reznicek et al. (2014) and calculated using the online Universal FQA Calculator (https://universalfqa.org; Freyman et al. 2015). The FQA tool assigns each native Michigan plant species a Coefficient of Conservatism value (C-value) ranging from 0 to 10 (Reznicek et al. 2014). The C-value reflects a given species’ fidelity to certa in ecological conditions. For individual sites a Mean C value () was generated (). Using the Mean C-value (), which is the average of the Coefficient of Conservatism values of species in that site, a Floristic Quality Index (FQI) for the entire site is calculated as follows:
where n is the number of species at the site. The Universal FQA Calculator generates a Native FQI and a Total FQI, the former based only on the native species present at the locality inventoried (as described above) and the latter on both native and non-native species (all non-native species have a C-value of 0).
Results and Discussion
This study provided a unique opportunity to examine and compare several distinct remnant floodplain sites along what was historically a single, nearly contiguous habitat meandering for roughly 26 miles through the Plaster Creek watershed. A total of 438 species of vascular plants, native and non-native, were recorded from seven floodplain habitats along Plaster Creek as it traverses from its headwaters in Gaines Township to the City of Grand Rapids where it eventually joins the Grand River. Individual sites ranged from having a flora of 115 species (26.7% of the total combined Plaster Creek flora) to 215 species (40.1% of the combined flora) (Tables 2 and 3).
Floristic Quality Assessment metrics for each Plaster Creek site, arranged in order from upstream to downstream. Estimates of area were obtained by using the Google Earth area calculator (DraftLogic 2022).
| Site | Area (ha) | Total FQI | Native FQI | Total Mean C | Total Species | Native Species | Non-Native Species |
|---|---|---|---|---|---|---|---|
| Crystal Springs | 2.14 | 31.1 | 33.5 | 2.9 | 115 | 97 (84.3%) | 18 (15.7%) |
| Paris Park | 17.9 | 47.4 | 50.0 | 3.7 | 164 | 149 (90.9%) | 15 (9.1%) |
| Wernlund Property | 2.28 | 30.4 | 34.7 | 2.8 | 118 | 93 (78.8%) | 25 (21.2%) |
| Covenant Park | 11.9 | 37.9 | 43.0 | 2.9 | 171 | 128 (74.9%) | 43 (25.1%) |
| Stanaback Park area | 21.8 | 48.4 | 52.8 | 3.3 | 215 | 174 (80.9%) | 41 (19.1%) |
| Ken-O-Sha Park | (9.5) | 52.7 | 58.4 | 3.6 | 214 | 176 (82.2%) | 38 (17.8%) |
| Madison Ave. Crossing | 4.91 | 34.6 | 42 | 2.8 | 153 | 110 (71.9%) | 43 (28.1%) |
List of species recorded in this study. The presence of a species at a given site is indicated by an X. The right-hand column gives the number of sites from which each species was recorded. State-listed rare species are indicated in boldface. The status of each listed species is indicated as follows: E = Endangered, T = Threatened, SC = Special Concern; locality withheld for E and T species.
| Species | Common Name | C-Value | Crystal Springs | Paris Park | Wemlund Property | Covenant Park | Stanaback Park | Ken-O-Sha Park | Madison Ave. | Number of Sites |
|---|---|---|---|---|---|---|---|---|---|---|
| Acer negundo L. | box-elder | 0 | X | X | X | X | X | X | X | 7 |
| Acer nigrum F. Michx. | black maple | 4 | X | X | X | X | X | 5 | ||
| Acer platanoides L. | Norway maple | 0 | X | 1 | ||||||
| Acer rubrum L. | red maple | 1 | X | X | X | X | X | 5 | ||
| Acer saccharinum L. | silver maple | 2 | X | X | X | X | X | X | X | 7 |
| Acer saccharum Marshall | sugar maple | 5 | X | X | X | X | X | X | X | 7 |
| Achillea millefolium L. | yarrow | 1 | X | 1 | ||||||
| Actaea pachypoda Elliott | doll’s eyes, white baneberry | 5 | X | 1 | ||||||
| Adiantum pedatum L. | maidenhair fern | 6 | X | 1 | ||||||
| Ageratina altissima (L.) R. M. King & H. Rob. | white snakeroot | 4 | X | X | X | 3 | ||||
| Agrimonia gryposepala Wallr. | tall agrimony | 2 | X | X | X | X | X | 5 | ||
| Agrimonia parviflora Aiton | swamp agrimony | 4 | X | 1 | ||||||
| Agrimonia pubescens Wallr. | soft agrimony | 5 | X | 3 | ||||||
| Agrostis stolonifera L. | creeping bent | 0 | X | X | X | 3 | ||||
| Ailanthus altissima (Mill.) Swingle | tree of heaven | 0 | X | 1 | ||||||
| Ajuga reptans L. | carpet bugle | 0 | X | 1 | ||||||
| Alisma triviale Pursh | northern water-plantain | 1 | X | X | X | 3 | ||||
| Alliaria petiolata (M. Bieb.) | ||||||||||
| Cavara & Grande | garlic mustard | 0 | X | X | X | X | X | X | X | 7 |
| Allium canadense L. | wild garlic | 4 | X | X | X | X | X | X | X | 7 |
| Allium tricoccum Aiton | wild leek | 5 | X | X | 2 | |||||
| Allium vineale L. | field garlic | 0 | X | X | X | X | 4 | |||
| Amphicarpaea bracteata (L.) Femald | hog-peanut | 5 | X | X | X | X | 4 | |||
| Anemone canadensis L. | Canada anemone | 4 | X | X | X | X | 4 | |||
| Anemone quinquefolia L. | wood anemone | 5 | X | 1 | ||||||
| Anemone virginiana L. | thimbleweed | 3 | X | 1 | ||||||
| Angelica atropurpurea L. | purplestem angelica | 6 | X | 2 | ||||||
| Apios americana Medik. | groundnut | 3 | X | X | 2 | |||||
| Apocynum androsaemifolium L. | spreading dogbane | 3 | X | X | X | 3 | ||||
| Apocynum cannabinum L. | Indian hemp | 3 | X | X | X | 3 | ||||
| Arabidopsis thaliana (L.) Heynh. | mouse-ear cress | 0 | X | X | 2 | |||||
| Arabis pycnocarpa M. Hopkins | hairy rock cress | 6 | X | 1 | ||||||
| Arctium minus Bernh. | common burdock | 0 | X | X | 2 | |||||
| Arisaema dracontium (L.) Schott | green dragon | 8 | X | X | X | X | 4 | |||
| Arisaema triphyllum (L.) Schott | Jack-in-the-pulpit | 5 | X | X | X | X | X | 5 | ||
| Asarum canadense L. | wild ginger | 5 | X | X | 2 | |||||
| Asclepias incarnata L. | swamp milkweed | 6 | X | X | X | 3 | ||||
| Asclepias syriaca L. | common milkweed | 1 | X | X | X | X | 4 | |||
| Asimina triloba (L.) Dunal | pawpaw | 9 | X | X | X | X | 4 | |||
| Asplenium platyneuron (L.) D. C. Eaton | ebony spleenwort | 2 | X | 1 | ||||||
| Barbarea vulgaris R. Br. | yellow rocket | 0 | X | X | X | X | X | 5 | ||
| Berberis aquifolium Pursh | Oregon-grape | 0 | X | 1 | ||||||
| Berberis thunbergii DC. | Japanese barberry | 0 | X | X | X | 3 | ||||
| Bidens comosa (A. Gray) Wiegand | swamp tickseed | 5 | X | 1 | ||||||
| Boechera canadensis (L.) Al-Shehbaz | sickle-pod | 7 | X | 1 | ||||||
| Boechera laevigata (Willd.) Al-Shehbaz | smooth bank cress | 5 | X | X | 2 | |||||
| Boehmeria cylindrica (L.) Sw. | false nettle | 5 | X | X | X | X | X | X | X | 7 |
| Bromus nottowayanus Fernald | satin brome | 7 | X | X | 2 | |||||
| Bromus pubescens Willd. | Canada brome | 5 | X | X | X | 3 | ||||
| Caltha palustris L. | marsh-marigold | 6 | X | X | 2 | |||||
| Calystegia sepium (Kit.) Griseb. | false bindweed | 0 | X | X | 2 | |||||
| Cardamine bulbosa (Muhl.) Britton, Sterns & Poggenb. | spring cress | 4 | X | X | X | 3 | ||||
| Cardamine concatenata (Michx.) O. Schwarz | cut-leaved toothwort | 5 | X | 1 | ||||||
| Cardamine douglassii Britton | pink spring cress | 6 | X | 1 | ||||||
| Cardamine hirsuta L. | hoary bitter cress | 0 | X | 1 | ||||||
| Cardamine impatiens L. | bitter cress | 0 | X | X | X | 3 | ||||
| Cardamine pensylvanica Willd. | Pennsylvania bitter cress | 1 | X | 1 | ||||||
| Carex albursina E. Sheld. | sedge | 5 | X | 1 | ||||||
| Carex amphibola Steud. | sedge | 8 | X | X | 2 | |||||
| Carex aquatilis Wahlenb. | sedge | 7 | X | 1 | ||||||
| Carex bebbii (L. H. Bailey) Femald | sedge | 4 | X | X | X | X | 4 | |||
| Carex blanda Dewey | sedge | 1 | X | X | X | X | X | X | 6 | |
| Carex bromoides Willd. | sedge | 6 | X | 1 | ||||||
| Carex cephaloidea (Dewey) Dewey | sedge | 5 | X | X | X | 3 | ||||
| Carex cephalophora Willd. | sedge | 3 | X | 1 | ||||||
| Carex crinita Lam. | sedge | 4 | X | X | X | 3 | ||||
| Carex cristatella Britton | ||||||||||
| Carex davisii | sedge Davis’ sedge | 3 | X | X | 2 | |||||
| Carex digitalis Willd. | sedge | 5 | X | 1 | ||||||
| Carex disperma Dewey | sedge | 10 | X | 1 | ||||||
| Carex echinodes (Fernald) P. Rothr., Reznicek & Hipp. | sedge | 5 | X | X | X | X | 4 | |||
| Carex emoryi Dewey | sedge | 7 | X | 1 | ||||||
| Carex gracilescens Steud. | sedge | 5 | X | X | 2 | |||||
| Carex gracillima Schwein. | sedge | 4 | X | X | X | X | 4 | |||
| Carex granularis Willd. | sedge | 2 | X | X | X | X | 4 | |||
| Carex grayi J. Carey | sedge | 6 | X | X | X | X | X | X | X | 7 |
| Carex grisea Wahlenb. | sedge | 3 | X | X | X | X | X | X | X | 7 |
| Carex hirtifolia Mack. | sedge | 5 | X | X | 2 | |||||
| Carex hystericina Willd. | sedge | 2 | X | X | 2 | |||||
| Carex intumescens Rudge | sedge | 3 | X | 1 | ||||||
| Carex jamesii Schwein. | James’ sedge | 8 | X | 1 | ||||||
| Carex lacustris Willd. | sedge | 6 | X | 1 | ||||||
| Carex laxiculmis Schwein. | sedge | 8 | X | X | 2 | |||||
| Carex laxiflora Lam. | sedge | 8 | X | X | 2 | |||||
| Carex lupulina Willd. | sedge | 4 | X | X | X | X | X | 5 | ||
| Carex lurida Wahlenb. | sedge | 3 | X | 1 | ||||||
| Carex molesta Mack. | sedge | 2 | X | X | 2 | |||||
| Carex normalis Mack. | sedge | 5 | X | X | X | 3 | ||||
| Carex ormostachya Wiegand | sedge | 5 | X | 1 | ||||||
| Carex pensylvanica Lam. | sedge | 4 | X | X | 2 | |||||
| Carex radiata (Wahlenb.) Small | straight-styled wood sedge | 2 | X | X | X | X | X | X | 6 | |
| Carex retrorsa Schwein. | sedge | 3 | X | 1 | ||||||
| Carex rosea Willd. | curly-styled wood sedge | 2 | X | X | X | 3 | ||||
| Carex scoparia Willd. | sedge | 4 | X | 1 | ||||||
| Carex sparganioides Willd. | sedge | 5 | X | X | X | X | 4 | |||
| Carex sprengelii Spreng. | sedge | 5 | X | X | X | 3 | ||||
| Carex stipata Willd. | sedge | 1 | X | X | X | X | X | X | 6 | |
| Carex stricta Lam. | sedge | 4 | X | X | 2 | |||||
| Carex swanii (Fernald) Mack. | sedge | 4 | X | X | X | 3 | ||||
| Carex tenera Dewey | sedge | 4 | X | 1 | ||||||
| Carex tribuloides Wahlenb. | sedge | 3 | X | 1 | ||||||
| Carex trichocarpa Willd. SC | hairy-fruited sedge | 8 | X | X | 2 | |||||
| Carex tuckermanii Dewey | sedge | 8 | X | 1 | ||||||
| Carex vulpinoidea Michx. | sedge | 1 | X | X | X | X | X | 5 | ||
| Carex woodii Dewey | sedge | 8 | X | 1 | ||||||
| Carpinus caroliniana Walter | hornbeam; blue-beech | 6 | X | X | X | X | X | X | 6 | |
| Carya cordiformis (Wang.) K. Koch | bittemut hickory | 5 | X | X | X | 3 | ||||
| Carya glabra (Mill.) Sweet | pignut hickory | 5 | X | 1 | ||||||
| Carya ovata (Mill.) K. Koch | shagbark hickory | 5 | X | X | X | X | X | 5 | ||
| Catalpa speciosa Warder | northern catalpa | 0 | X | X | X | X | 4 | |||
| Caulophyllum thalictroides (L.) Michx. | blue cohosh | 5 | X | 1 | ||||||
| Celastrus orbiculatus Thunb. | oriental bittersweet | 0 | X | X | X | 3 | ||||
| Celtis occidentalis L. | hackberry | 5 | X | X | X | X | X | 5 | ||
| Centaurea stoebe L. | spotted knapweed | 0 | X | 1 | ||||||
| Cephalanthus occidentalis L. | buttonbush | 7 | X | X | X | X | 4 | |||
| Cerastium fontanum Baumg. | mouse-ear chickweed | 0 | X | X | 2 | |||||
| Cerastium nutans Raf. | nodding chickweed | 4 | X | 1 | ||||||
| Cercis canadensis L. | redbud | 8 | X | X | X | 3 | ||||
| Chasmanthium latifolium (Michx.) H. O. Yates | wild oats | 0 | X | 1 | ||||||
| Chelone glabra L. | turtlehead | 7 | X | X | 2 | |||||
| Chrysosplenium americanum Hook. | golden saxifrage | 6 | X | 1 | ||||||
| Cicuta maculata L. | water hemlock | 4 | X | X | X | X | 4 | |||
| Cinna arundinacea L. | wood reedgrass | 7 | X | X | X | X | 4 | |||
| Circaea canadensis (L.) Hill | enchanters-nightshade | 2 | X | X | X | X | X | X | 6 | |
| Cirsium arvense (L.) Scop. | Canada thistle | 0 | X | X | X | X | 4 | |||
| Cirsium muticum Michx. | swamp thistle | 6 | X | 1 | ||||||
| Cirsium vulgare (Savi) Ten. | bull thistle | 0 | X | X | X | 3 | ||||
| Claytonia virginica L. | spring-beauty | 4 | X | X | X | X | X | X | 6 | |
| Clematis virginiana L. | virgin’s bower | 4 | X | X | X | 3 | ||||
| Clinopodium vulgare L. | wild-basil | 3 | X | 1 | ||||||
| Comarum palustre L. | marsh cinquefoil | 7 | X | 1 | ||||||
| Conopholis americana (L.) Wallr. | American cancer-root | 10 | X | 1 | ||||||
| Convallaria majalis L. | lily of the valley | 0 | X | X | 2 | |||||
| Cornus alternifolia L. f. | alternate-leaved dogwood | 5 | X | 1 | ||||||
| Cornus amomum Mill. | silky dogwood | 2 | X | 1 | ||||||
| Cornus florida L. | flowering dogwood | 8 | X | 1 | ||||||
| Cornus foemina Mill. | gray dogwood | 1 | X | X | 2 | |||||
| Cornus sericea L. | red-osier | 2 | X | X | 2 | |||||
| Crataegus punctata Jacq. | dotted hawthorn | 1 | X | X | X | 3 | ||||
| Crataegus succulenta Link | hawthorn | 5 | X | 1 | ||||||
| Cryptotaenia canadensis (L.) DC. | honewort | 2 | X | X | X | X | X | X | 6 | |
| Cuscuta gronovii Roem. & Schult. | common dodder | 3 | X | X | 2 | |||||
| Cynoglossum officinale L. | hound’s tongue | 0 | X | 1 | ||||||
| Cystopteris bulbifera (L.) Bernh. | bulblet fern | 5 | X | 1 | ||||||
| Cystopteris protrusa (Wealth.) Blasdell | fragile fern | 5 | X | 1 | ||||||
| Cystopteris tenuis (Michx.) Desv. | fragile fern | 5 | X | 1 | ||||||
| Dactylis glomerata L. | orchard grass | 0 | X | X | X | X | 4 | |||
| Danthonia spicata (L.) Roem. & Schult. | poverty grass; oatgrass | 4 | X | 1 | ||||||
| Daucus carota L. | Queen Anne’s lace | 0 | X | 1 | ||||||
| Dianthus armeria L. | Deptford pink | 0 | X | 1 | ||||||
| Diarrhena obovata (Gleason) Brandenburg | beak grass | 9 | X | X | X | 3 | ||||
| Dichanthelium clandestinum (L.) Gould | panic grass | 3 | X | X | 2 | |||||
| Dichanthelium implicatum (Scribn.) Kerguélen | panic grass | 3 | X | 1 | ||||||
| Dichanthelium latifolium (L.) Harvill | broad-leaved panic grass | 5 | X | 1 | ||||||
| Dichanthelium lindheimeri (Nash) Gould | panic grass | 8 | X | 1 | ||||||
| Digitaria sanguinalis (L.) Scop. | hairy crab grass | 0 | X | 1 | ||||||
| Dioscorea villosa L. | wild yam | 4 | X | X | X | |||||
| Dipsacus fullonum L. | wild teasel | 0 | X | 1 | ||||||
| Dryopteris carthusiana (Vill.) H. P. Fuchs | spinulose woodfem | 5 | X | X | X | X | 4 | |||
| Echinochloa crusgalli (L.) P. Beauv. | barnyard grass | 0 | X | X | 2 | |||||
| Echinocystis lobata (Michx.) Torr. & A. Gray | wild-cucumber | 2 | X | 1 | ||||||
| Elaeagnus umbellata Thunb. | autumn-olive | 0 | X | X | X | X | X | 5 | ||
| Elymus hystrix L. | bottlebrush grass | 5 | X | X | X | 3 | ||||
| Elymus riparius Wiegand | riverbank wild-rye | 8 | X | X | X | 3 | ||||
| Elymus villosus Willd. | silky wild-rye | 5 | X | X | X | 3 | ||||
| Elymus virginicus L. | Virginia wild-rye | 4 | X | X | X | X | X | X | X | 7 |
| Enemion biternatum Raf. | false rue anemone | 8 | X | X | X | X | 4 | |||
| Epilobium parviflorum Schreb. | willow herb | 0 | X | 1 | ||||||
| Epipactis helleborine (L.) Crantz | helleborine | 0 | X | X | X | 3 | ||||
| Equisetum arvense L. | common horsetail | 0 | X | X | X | X | 4 | |||
| Equisetum hyemale L. | scouring rush | 2 | X | 1 | ||||||
| Equisetum laevigatum A.Braun | smooth scouring rush | 2 | X | 1 | ||||||
| Erigeron annuus (L.) Pers. | daisy fleabane | 0 | X | X | X | X | X | X | 6 | |
| Erigeron philadelphicus L. | common fleabane | 2 | X | X | 2 | |||||
| Erigeron strigosus Willd. | daisy fleabane | 4 | X | 1 | ||||||
| Erythronium albidum Ker Gawl. | white trout lily | 7 | X | 1 | ||||||
| Erythronium americanum Ker Gawl. | yellow trout lily | 5 | X | X | X | 3 | ||||
| Euonymus alatus (Thunb.) Siebold | winged euonymus | 0 | X | X | 2 | |||||
| Euonymus atropurpureus Jacq. - SC | wahoo, burning-bush | 8 | X | 1 | ||||||
| Euonymus obovatus Nutt. | running strawberry-bush | 5 | X | X | 2 | |||||
| Eupatorium perfoliatum L. | boneset | 4 | X | 1 | ||||||
| Eutrochium maculatum (L.) E. E. Lamont | Joe-pye-weed | 4 | X | X | X | X | X | 5 | ||
| Fagus grandifolia Ehrh. | American beech | 6 | X | X | X | X | 4 | |||
| Fallopia convolvulus (L.) Á. Löve | black bindweed | 0 | X | X | X | 3 | ||||
| Festuca subverticillata (Pers.) E. B. Alexeev | nodding fescue | 5 | X | X | 2 | |||||
| Festuca trachyphylla (Hack.) Krajina | sheep fescue | 0 | X | 1 | ||||||
| Ficaria verna Huds. | lesser celandine | 0 | X | X | 2 | |||||
| Floerkea proserpinacoides Willd. | false mermaid | 7 | X | X | 2 | |||||
| Fragaria virginiana Mill. | wild strawberry | 2 | X | X | X | X | 4 | |||
| Frangula alnus Mill. | glossy buckthorn | 0 | X | 1 | ||||||
| Fraxinus americana L. | white ash | 5 | X | X | X | 3 | ||||
| Fraxinus nigra Marshall | black ash | 6 | X | X | 2 | |||||
| Fraxinus pennsylvanica Marshall | red ash | 2 | X | X | X | X | X | X | X | 7 |
| Galium aparine L. | annual bedstraw | 0 | X | X | X | X | X | X | 6 | |
| Galium asprellum Michx. | rough bedstraw | 5 | X | 1 | ||||||
| Galium circaezans Michx. | white wild licorice | 4 | X | X | 2 | |||||
| Galium obtusum Bigelow | wild madder | 5 | X | X | X | X | X | 5 | ||
| Galium palustre L. | marsh bedstraw | 3 | X | X | 2 | |||||
| Geranium maculatum L. | wild geranium | 4 | X | X | X | X | 4 | |||
| Geranium robertianum L. | herb Robert | 3 | X | 1 | ||||||
| Geum aleppicum Jacq. | yellow avens | 3 | X | X | 2 | |||||
| Geum canadense Jacq. | white avens | 1 | X | X | X | X | X | X | X | 7 |
| Geum vernum (Raf.) T. & G. | spring avens | 4 | X | X | X | 3 | ||||
| Glechoma hederacea L. | ground-ivy | 0 | X | X | X | X | X | X | 6 | |
| Glyceria striata (Lam.) Hitchc. | fowl manna grass | 4 | X | X | X | X | X | X | X | 7 |
| Hackelia virginiana (L.) I. M. Johnst. | beggars lice | 1 | X | X | X | X | X | 5 | ||
| Hamamelis virginiana L. | witch-hazel | 5 | X | X | 2 | |||||
| Heracleum maximum Bartram | cow-parsnip | 3 | X | 1 | ||||||
| Hesperis matronalis L. | dames rocket | 0 | X | X | X | X | X | X | X | 7 |
| Hieracium aurantiacum L. | orange hawkweed | 0 | X | 1 | ||||||
| Hieracium caespitosum Dumort. | king devil | 0 | X | X | 2 | |||||
| Hydrophyllum canadense L. | Canada waterleaf | 7 | X | 1 | ||||||
| Hydrophyllum virginianum L. | Virginia waterleaf | 4 | X | 1 | ||||||
| Hylodesmum nudiflorum (L.) H. Ohashi & R. R. Mill | naked tick-trifoil | 7 | X | 1 | ||||||
| Hypericum perforatum L. | common St. -ohns-wort | 0 | X | 1 | ||||||
| Hypericum prolificum L. | shrubby St. John’s-wort | 5 | X | 1 | ||||||
| Hypericum punctatum Lam. | spotted St. John’s-wort | 4 | X | X | 2 | |||||
| Hypopitys monotropa Crantz | pinesap | 6 | X | 1 | ||||||
| Ilex verticillata (L.) A. Gray | Michigan holly | 5 | X | 1 | ||||||
| Impatiens capensis Meerb. | spotted touch-me-not | 2 | X | X | X | X | X | X | 6 | |
| Iris pseudacorus L. | yellow flag | 0 | X | X | X | 3 | ||||
| Iris virginica L. | Southern blue flag | 5 | X | X | X | X | X | X | 6 | |
| Juglans nigra L. | black walnut | 5 | X | X | X | X | X | X | X | 7 |
| Juncus dudleyi Wiegand | Dudleys rush | 1 | X | X | 2 | |||||
| Juncus effusus L. | soft-stemmed rush | 3 | X | X | 2 | |||||
| Juncus tenuis Willd. | path rush | 1 | X | X | X | X | X | X | 6 | |
| Laportea canadensis (L.) Wedd. | wood nettle | 4 | X | X | X | X | X | 5 | ||
| Lapsana communis L. | nipplewort | 0 | X | 1 | ||||||
| Leersia oryzoides (L.) Sw. | cut grass | 3 | X | X | X | 3 | ||||
| Leersia virginica Willd. | white grass | 5 | X | X | X | X | X | 5 | ||
| Lemna minor L. | common duckweed | 5 | X | X | X | 3 | ||||
| Leonurus cardiaca L. | motherwort | 0 | X | X | X | 3 | ||||
| Leucanthemum vulgare Lam. | ox-eye daisy | 0 | X | X | 2 | |||||
| Ligustrum obtusifolium | border privet | 0 | X | 1 | ||||||
| Siebold & Zucc. | ||||||||||
| Ligustrum vulgare L. | common privet | 0 | X | 1 | ||||||
| Lilium michiganense Farw. | Michigan lily | 5 | X | X | 2 | |||||
| Lindera benzoin (L.) Blume | spicebush | 7 | X | X | X | X | X | 5 | ||
| Lithospermum latifolium Michx. - SC | broadleaved puccoon | 10 | X | 1 | ||||||
| Lobelia cardinalis L. | cardinal-flower | 7 | X | X | X | X | X | 5 | ||
| Lobelia siphilitica L. | great blue lobelia | 4 | X | 1 | ||||||
| Lonicera ×bella Zabel | hybrid honeysuckle | 0 | X | X | X | 3 | ||||
| Lonicera maackii (Rupr.) Herder | Armur honeysuckle | 0 | X | 1 | ||||||
| Lonicera morrowii A. Gray | Morrow honeysuckle | 0 | X | X | X | 3 | ||||
| Lonicera tatarica L. | Tatarian honeysuckle | 0 | X | 1 | ||||||
| Ludwigia palustris (L.) Elliott | water-purslane | 4 | X | 1 | ||||||
| Luzula acuminata Raf. | hairy wood rush | 5 | X | 1 | ||||||
| Lycopus americanus Muhl. | common water horehound | 2 | X | X | X | X | 4 | |||
| Lycopus uniflorus Michx. | northern bugle weed | 2 | X | 1 | ||||||
| Lysimachia ciliata L. | fringed loosestrife | 4 | X | X | X | X | X | 5 | ||
| Lysimachia nummularia L. | moneywort | 0 | X | X | X | X | X | X | 6 | |
| Lysimachia thyrsiflora L. | tufted loosestrife | 6 | X | 1 | ||||||
| Lythrum salicaria L. | purple loosestrife | 0 | X | X | X | 3 | ||||
| Maianthemum canadense Desf. | Canada Mayflower | 4 | X | 1 | ||||||
| Maianthemum racemosum (L.) Link | false spikenard | 5 | X | X | 2 | |||||
| Maianthemum stellatum (L.) Link | starry false Solomon-seal | 5 | X | 1 | ||||||
| Malus pumila Mill. | apple | 0 | X | X | 2 | |||||
| Matteuccia struthiopteris (L) Todaro | ostrich fern | 3 | X | X | 2 | |||||
| Medicago lupulina L. | black medick | 0 | X | X | X | X | 4 | |||
| Melilotus albus Medik. | white sweet-clover | 0 | X | 1 | ||||||
| Melissa officinalis L. | lemon-balm | 0 | X | 1 | ||||||
| Menispermum canadense L. | moonseed | 5 | X | X | X | X | 4 | |||
| Mentha canadensis L. | wild mint | 3 | X | X | X | 3 | ||||
| Mertensia virginica (L.) Pers. - E | Virginia bluebells | 10 | 3 | |||||||
| Micranthes pensylvanica (L.) Haw. | swamp saxifrage | 10 | X | 1 | ||||||
| Mimulus ringens L. | monkey-flower | 5 | X | X | X | X | 4 | |||
| Moehringia lateriflora (L.) Fenzl | wood sandwort | 5 | X | 1 | ||||||
| Monarda fistulosa L. | wild-bergamot | 2 | X | X | X | X | 4 | |||
| Monotropa uniflora L. | Indian-pipe | 5 | X | X | 2 | |||||
| Morus alba L. | white mulberry | 0 | X | X | X | X | X | 5 | ||
| Nasturtium officinale W. T. Aiton | watercress | 0 | X | X | 2 | |||||
| Nepeta cataria L. | catnip | 0 | X | 1 | ||||||
| Onoclea sensibilis L. | sensitive fern | 2 | X | X | X | X | X | 5 | ||
| Osmorhiza claytonii (Michx.) C. B. Clarke | hairy sweet-cicely | 4 | X | X | 2 | |||||
| Osmunda regalis L. | royal fern | 5 | X | 1 | ||||||
| Ostrya virginiana (Mill.) K. Koch. | ironwood; hop-hornbeam | 5 | X | X | X | X | X | X | 6 | |
| Oxalis dillenii Jacq. | common yellow wood-sorrel | 0 | X | X | X | 3 | ||||
| Oxalis stricta L. | yellow wood-sorrel | 0 | X | X | X | X | 4 | |||
| Packera aurea (L.) Á. Löve & D. Löve | golden ragwort | 5 | X | X | 2 | |||||
| Parietaria pensylvanica Willd. | pellitory | 2 | X | 1 | ||||||
| Parthenocissus quinquefolia (L.) Planch. | Virginia creeper | 5 | X | X | X | X | X | X | 6 | |
| Penstemon digitalis Nutt. | floxglove beard-tongue | 2 | X | 1 | ||||||
| Penthorum sedoides L. | ditch stonecrop | 3 | X | 1 | ||||||
| Persicaria hydropiperoides (Michx.) Small | mild water-pepper | 5 | X | X | X | 3 | ||||
| Persicaria longiseta (Bruijn) Kitag. | creeping smartweed | 0 | X | X | 2 | |||||
| Persicaria maculosa Gray | lady’s-thumb | 0 | X | X | X | 3 | ||||
| Persicaria punctata (Elliott) Small | smartweed | 5 | X | X | 2 | |||||
| Persicaria sagittata (L.) H. Gross | arrow-leaved tear-thumb | 5 | X | X | 2 | |||||
| Persicaria virginiana (L.) Gaertn. | jumpseed | 4 | X | X | X | X | X | X | 6 | |
| Phalaris arundinacea L. | reed canary grass | 0 | X | X | X | X | X | 5 | ||
| Phellodendron amurense Rupr. | Amur cork-tree | 0 | X | 1 | ||||||
| Phlox divaricata L. | wild blue phlox | 5 | X | X | 2 | |||||
| Phryma leptostachya L. | lopseed | 4 | X | X | 2 | |||||
| Physalis heterophylla Nees | clammy ground-cherry | 3 | X | 1 | ||||||
| Physocarpus opulifolius (L.) Maxim. | ninebark | 4 | X | X | 2 | |||||
| Phytolacca americana L. | pokeweed | 2 | X | X | X | 3 | ||||
| Pilea pumila (L.) A. Gray | clearweed | 5 | X | X | 2 | |||||
| Plantago major L. | common plantain | 0 | X | X | X | 3 | ||||
| Plantago rugelii Decne. | red-stalked plantain | 0 | X | 1 | ||||||
| Platanus occidentalis L. | sycamore | 7 | X | X | X | X | X | X | 6 | |
| Poa alsodes A. Gray | bluegrass | 9 | X | 1 | ||||||
| Poa compressa L. | Canadda bluegrass | 0 | X | 1 | ||||||
| Poa languida Hitchc. | bluegrass | 6 | X | 1 | ||||||
| Poa palustris L. | fowl meadow grass | 3 | X | X | X | 3 | ||||
| Poa pratensis L. | Kentucky bluegrass | 0 | X | X | X | 3 | ||||
| Poa saltuensis Fernald & Wiegand | bluegrass | 5 | X | 1 | ||||||
| Poa sylvestris A. Gray | woodland bluegrass | 8 | X | 1 | ||||||
| Poa trivialis L. | bluegrass | 0 | X | X | X | 3 | ||||
| Podophyllum peltatum L. | May-apple | 3 | X | X | X | X | X | X | 6 | |
| Polygonatum biflorum (Walter) Elliott | Solomon-seal | 4 | X | 1 | ||||||
| Polystichum acrostichoides (Michx.) Schott. | Christmas fern | 6 | X | 1 | ||||||
| Populus deltoides Marshall | cottonwood | 1 | X | X | X | X | X | X | 6 | |
| Potamogeton pusillus L. | small pondweed | 4 | X | 1 | ||||||
| Potentilla indica (Andrews) T. Wolf | Indian-strawberry | 0 | X | X | 2 | |||||
| Potentilla recta L. | rough-fruited cinquefoil | 0 | X | 1 | ||||||
| Potentilla simplex Michx. | old-field cinquefoil | 2 | X | 1 | ||||||
| Proserpinaca palustris L. | mermaid-weed | 6 | X | 1 | ||||||
| Prunella vulgaris L. | self-heal | 0 | X | X | X | X | X | 5 | ||
| Prunus avium (L.) L. | sweet cherry | 0 | X | 1 | ||||||
| Prunus serotina Ehrh. | wild black cherry | 2 | X | X | X | X | X | X | X | 7 |
| Prunus virginiana L. | choke cherry | 2 | X | X | X | 3 | ||||
| Ptelea trifoliata L. | hop-tree | 4 | X | X | 2 | |||||
| Quercus alba L. | white oak | 5 | X | X | X | 3 | ||||
| Quercus bicolor Willd. | swamp white oak | 8 | X | X | X | X | X | X | X | 7 |
| Quercus macrocarpa Michx. | bur oak | 5 | X | X | X | X | X | X | 6 | |
| Quercus muehlenbergii Engelm. | chinquapin oak | 5 | X | X | X | X | X | 5 | ||
| Quercus rubra L. | red oak | 5 | X | X | X | X | X | 5 | ||
| Quercus velutina Lam. | black oak | 5 | X | 1 | ||||||
| Ranunculus abortivus L. | small-flowered buttercup | 0 | X | 1 | ||||||
| Ranunculus bulbosus L. | bulbous buttercup | 0 | X | X | 2 | |||||
| Ranunculus hispidus Michx. | swamp buttercup | 5 | X | X | X | X | X | X | 6 | |
| Ranunculus repens L. | creeping buttercup | 0 | X | 1 | ||||||
| Ranunculus sceleratus L. | cursed crowfoot | 1 | X | 1 | ||||||
| Rhamnus alnifolia L’Her. | alder-leaved buckthorn | 8 | X | 1 | ||||||
| Rhamnus cathartica L. | common buckthorn | 0 | X | X | X | 3 | ||||
| Rhodotypos scandens (Thunb.) Makino | jetbead | 0 | X | X | 2 | |||||
| Rhus glabra L. | smooth sumac | 2 | X | 1 | ||||||
| Rhus typhina L. | staghorn sumac | 2 | X | 1 | ||||||
| Ribes americanum Mill. | wild black currant | 6 | X | X | X | 3 | ||||
| Ribes cynosbati L. | wild gooseberry | 4 | X | X | X | X | X | 5 | ||
| Robinia pseudoacacia L. | black locust | 0 | X | 1 | ||||||
| Rorippa palustris (L.) Besser | yellow cress | 1 | X | X | 2 | |||||
| Rorippa sylvestris (L.) Besser | creeping yellow cress | 0 | X | 1 | ||||||
| Rosa multiflora Murray | multiflora rose | 0 | X | X | X | X | X | X | 6 | |
| Rubus allegheniensis Porter | common blackberry | 1 | X | X | X | X | 4 | |||
| Rubus flagellaris Willd. | northern dewberry | 1 | X | X | 2 | |||||
| Rubus occidentalis L. | black raspberry | 1 | X | X | X | X | X | 5 | ||
| Rubus pensilvanicus Poir. | dewberry | 2 | X | 1 | ||||||
| Rudbeckia hirta L. | black-eyed Susan | 1 | X | 1 | ||||||
| Rudbeckia laciniata L. | cut-leaf coneflower | 6 | X | X | 2 | |||||
| Rudbeckia triloba L. | three-lobed coneflower | 5 | X | 1 | ||||||
| Rumex crispus L. | curly dock | 0 | X | X | X | X | X | 5 | ||
| Rumex obtusifolius L. | bitter dock | 0 | X | X | X | X | X | X | 6 | |
| Rumex verticillatus L. | water dock | 7 | X | X | X | X | 4 | |||
| Sagittaria cuneata E. Sheld. | arum-leaved arrowhead | 6 | X | 1 | ||||||
| Sagittaria latifolia Willd. | wapato, common arrowhead | 4 | X | 1 | ||||||
| Salix alba L. | white willow | 0 | X | X | 2 | |||||
| Salix amygdaloides Andersson | peach-leaved willow | 3 | X | X | 2 | |||||
| Salix discolor Muhl. | pussy willow | 1 | X | 1 | ||||||
| Salix eriocephala Michx. | willow | 2 | X | 1 | ||||||
| Salix exigua Nutt. | sandbar willow | 1 | X | X | 2 | |||||
| Salix nigra Marshall | black willow | 5 | X | 1 | ||||||
| Salix sericea Marshall | silky willow | 6 | X | X | 2 | |||||
| Sambucus canadensis L. | elderberry | 3 | X | X | 2 | |||||
| Samolus parviflorus Raf. | water-pimpernel | 5 | X | X | 2 | |||||
| Sanguinaria canadensis L. | bloodroot | 5 | X | X | 2 | |||||
| Sanicula canadensis L. | black snakeroot | 8 | X | 1 | ||||||
| Sanicula odorata (Raf.) Pryer & Phillippe | black snakeroot | 2 | X | X | X | 3 | ||||
| Saponaria officinalis L. | bouncing bet | 0 | X | 1 | ||||||
| Sassafras albidum (Nutt.) Nees | sassafras | 5 | X | X | 2 | |||||
| Schoenoplectus tabernaemontani (C. C. Gmel.) Palla | softstem bulrush | 4 | X | 1 | ||||||
| Scirpus atrovirens Willd. | bulrush | 3 | X | X | X | X | 4 | |||
| Scirpus expansus Fernald | bulfush | 5 | X | 1 | ||||||
| Scirpus hattorianus Makino | mosquito bulrush | 3 | X | 1 | ||||||
| Scirpus pendulus Muhl. | bulrush | 3 | X | 1 | ||||||
| Scrophularia marilandica L. | late figwort | 5 | X | X | 2 | |||||
| Scutellaria lateriflora L. | mad-dog skullcap | 5 | X | X | X | X | 4 | |||
| Silene latifolia Poir. | white campion | 0 | X | X | X | 3 | ||||
| Sisyrinchium angustifolium Mill. | stout blue-eyed-grass | 4 | X | X | X | X | X | 5 | ||
| Sium suave Walter | water-parsnip | 5 | X | 1 | ||||||
| Smilax ecirrata (Kunth) S. Watson | upright carrion-flower | 6 | X | 1 | ||||||
| Smilax hispida Raf. | bristly greenbrier | 5 | X | X | X | 3 | ||||
| Solanum carolinense L. | horse-nettle | 0 | X | 1 | ||||||
| Solanum dulcamara L. | bittersweet nightshade | 0 | X | X | X | X | 4 | |||
| Solanum ptychanthum Dunal | black nightshade | 1 | X | X | 2 | |||||
| Solidago canadensis L. | Canada goldenrod | 1 | X | X | X | 3 | ||||
| Solidago gigantea Aiton | late goldenrod | 3 | X | X | X | 3 | ||||
| Sparganium eurycarpum Engelm. | common bur-reed | 5 | X | X | 2 | |||||
| Sphenopholis intermedia (Rydb.) Rydb. | slender wedgegrass | 4 | X | X | X | X | 4 | |||
| Stachys hispida Pursh | hedge-nettle | 5 | X | X | 2 | |||||
| Stachys tenuifolia Willd. | smooth hedge nettle | 5 | X | 1 | ||||||
| Staphylea trifolia L. | bladdernut | 9 | X | X | X | 3 | ||||
| Symphyotrichum lateriflorum (L.) Á. Löve & D. Löve | calico aster | 2 | X | 1 | ||||||
| Symphyotrichum novae-angliae (L.) G. L. Nesom | New England aster | 3 | X | 1 | ||||||
| Symphyotrichum ontarionis (Wiegand) G. L. Nesom | Lake Ontario aster | 6 | X | 1 | ||||||
| Symphyotrichum pilosum (Willd.) G. L. Nesom | hairy aster, frost aster | 1 | X | 1 | ||||||
| Symphyotrichum puniceum (L.) Á. Löve & D. Löve | swamp aster | 5 | X | 1 | ||||||
| Symplocarpus foetidus (L.) Nutt. | skunk-cabbage | 6 | X | X | X | X | X | 5 | ||
| Taraxacum officinale F. H. Wigg. | common dandelion | 0 | X | X | X | X | 4 | |||
| Teucrium canadense L. | wood-sage | 4 | X | X | X | X | 4 | |||
| Thalictrum dasycarpum Fisch. & Ave-Lall. | purple meadow-rue | 3 | X | X | X | X | X | X | 6 | |
| Thalictrum dioicum L. | early meadow-rue | 6 | X | X | X | 3 | ||||
| Thalictrum thalictroides (L.) Eames & B. Boivin | rue-anemone | 8 | X | X | 2 | |||||
| Thelypteris noveboracensis (L.) Nieuwl. | New York fern | 5 | X | 1 | ||||||
| Thelypteris palustris Schott | marsh fern | 2 | X | 1 | ||||||
| Thuja occidentalis L. | white cedar | 4 | X | 1 | ||||||
| Tilia americana L. | basswood | 5 | X | X | X | X | X | X | X | 7 |
| Torilis japonica (Houtt.) DC. | hedge-parsley | 0 | X | X | 2 | |||||
| Toxicodendron radicans (L.) Kuntze | poison-ivy | 2 | X | X | X | X | X | X | X | 7 |
| Tradescantia ohiensis Raf. | common spiderwort | 5 | X | 1 | ||||||
| Tragopogon pratensis L. | common goats beard | 0 | X | X | X | 3 | ||||
| Trifolium hybridum L. | alsike clother | 0 | X | 1 | ||||||
| Trifolium repens L. | white clover | 0 | X | X | X | 3 | ||||
| Trillium grandiflorum (Michx.) Salisb. | common trillium | 5 | X | X | X | X | 4 | |||
| Typha angustifolia L. | narrow-leaved cattail | 0 | X | 1 | ||||||
| Ulmus americana L. | American elm | 1 | X | X | X | X | X | X | X | 7 |
| Urtica dioica L. | stinging nettle | 1 | X | X | X | X | X | X | X | 7 |
| Uvularia grandiflora Sm. | bellwort | 5 | X | 1 | ||||||
| Verbascum blattaria L. | moth mullein | 0 | X | 1 | ||||||
| Verbascum densiflorum Bertol. | mullein | 0 | X | 1 | ||||||
| Verbascum thapsus L. | common mullein | 0 | X | X | X | 3 | ||||
| Verbena urticifolia L. | white vervain | 4 | X | X | X | X | X | X | X | 7 |
| Vernonia missurica Raf. | Missouri ironweed | 4 | X | X | X | 3 | ||||
| Veronica hederifolia L. | ivy-leaved speedwell | 0 | X | 1 | ||||||
| Veronica serpyllifolia L. | thyme-leaved veronica | 0 | X | 1 | ||||||
| Viburnum acerifolium L. | maple-leaved viburnum | 6 | X | X | 2 | |||||
| Viburnum lentago L. | nannyberry | 4 | X | X | 2 | |||||
| Viburnum opulus L. | European highbush-cranberry | 0 | X | 1 | ||||||
| Viburnum plicatum Thunb. | Japanese snowball | 0 | X | 1 | ||||||
| Viburnum trilobum Marshall | American high-bush-cranberry | 5 | X | 1 | ||||||
| Vinca minor L. | periwinkle | 0 | X | 1 | ||||||
| Vincetoxicum nigrum (L.) Pers. | black swallow-wort | 0 | X | X | 2 | |||||
| Viola canadensis L. | Canada violet | 5 | X | X | 2 | |||||
| Viola cucullata Aiton | marsh violet | 5 | X | 1 | ||||||
| Viola pubescens Aiton | yellow violet | 4 | X | X | X | X | 4 | |||
| Viola sororia Willd. | common blue violet | 1 | X | X | X | X | X | X | 6 | |
| Viola striata Aiton | cream violet | 5 | X | X | X | X | X | X | X | 7 |
| Vitis aestivalis Michx. | summer grape | 6 | X | 1 | ||||||
| Vitis riparia Michx. | river-bank grape | 3 | X | X | X | X | X | X | X | 7 |
| Zanthoxylum americanum Mill. | prickly ash | 3 | X | X | X | 3 | ||||
| Total number of species/site | 115 | 164 | 118 | 172 | 215 | 215 | 154 |
Individual Site Assessments
Among the seven floodplains inventoried in 2012–2022 (Figure 1), the Total FQI values ranged from a low of 30.4 (Total Mean C = 2.8) at the Wernlund Property to a high of 52.7 (Total Mean C = 3.6) at the Ken-O-Sha Park floodplain. The FQA metrics for each of these seven sites are given in Table 2, and Table 3 lists all species recorded (collectively) by individual site. Table 4 lists all species reported by Cole from the 1890s, as well as those from our study, that have a C-value of 8–10 (indicating a present-day high level of fidelity to a narrow range of ecological conditions); the state listing status is also indicated.
Species having a C-value of 8–10, indicating a high level of fidelity to a narrow range of undisturbed ecological conditions, among those collected and reported by Emma Cole from along Plaster Creek and those collected in the current study. An X indicates the presence of a species in each case. The state status of listed species, which are in boldface, is indicated as follows: E = Endangered; T = Threatened; SC = Special Concern.
| Species | State Listing Status | C-Value | Emma Cole 1890s | Current Study |
|---|---|---|---|---|
| Carex disperma | 10 | X | ||
| Carex prairea | 10 | X | ||
| Carex stipata | 10 | X | ||
| Conioselinum chinense | 10 | X | ||
| Conopholis americana | 10 | X | ||
| Filipendula rubra | T | 10 | X | |
| Hypericum kalmianum | 10 | X | ||
| Lithospermum latifolium | SC | 10 | X | |
| Lysimachia quadriflora | 10 | X | ||
| Mertensia virginica | E | 10 | X | X |
| Micranthes pensylvanica | 10 | X | ||
| Trillium nivale | T | 10 | X | |
| Asimina triloba | 9 | X | X | |
| Carex tetanica | 9 | X | ||
| Cypripedium reginae | 9 | X | ||
| Diarrhena obovata | 9 | X | ||
| Deschampsia cespitosa | 9 | X | ||
| Jeffersonia diphylla | SC | 9 | X | |
| Morus rubra | T | 9 | X | |
| Poa alsodes | 9 | X | ||
| Rumex orbiculatus | 9 | X | ||
| Salix candida | 9 | X | ||
| Salix myricoides | 9 | X | ||
| Staphylea trifolia | 9 | X | ||
| Arisaema dracontium | 8 | X | X | |
| Carex amphibola | 8 | X | ||
| Carex jamesii | 8 | X | ||
| Carex laxiculmis | 8 | X | ||
| Carex laxiflora | 8 | X | X | |
| Carex trichocarpa | SC | 8 | X | |
| Carex tuckermanii | 8 | X | ||
| Carex woodii | 8 | X | X | |
| Cercis canadensis | 8 | X | X | |
| Chaerophyllum procumbens | 8 | X | ||
| Cornus florida | 8 | X | ||
| Dichanthelium lindheimeri | 8 | X | ||
| Elymus riparius | 8 | X | ||
| Enemion biternatum | 8 | X | ||
| Eriophorum virginicum | 8 | X | ||
| Euonymus atropurpureus | SC | 8 | X | |
| Menyanthes trifoliata | 8 | X | ||
| Orobanche uniflora | 8 | X | ||
| Poa sylvestris | 8 | X | ||
| Quercus bicolor | 8 | X | ||
| Rhamnus alnifolia | 8 | X | ||
| Sanicula canadensis | 8 | X | ||
| Valerianella chenopodiifolia | 8 | X | ||
| TOTALS | 8 | 25 | 27 |
Crystal Springs (42° 50.850′N, 85°35.497′W)
A total of 115 species, of which 84.3% are native, were recorded at Crystal Springs (Figure 2). The Floristic Quality Assessment (Table 2) showed a Total FQI of 31.1 and a Native FQI of 33.5. Thus, this community ranks as average quality with respect to floristic value to the state (Table 1). The Total Mean C was 2.9. Only three species have high-fidelity C-values, all C-8: false rue anemone (Enemion biternatum), swamp white oak (Quercus bicolor), and wahoo (Euonymus atropurpureus). Overall, this site was the most degraded and had the lowest species richness of our seven sites.
Paris Park (42° 51.348′N, 85° 35.075′W)
A total of 164 species, of which 90.9% are native, were recorded at Paris Park (Figure 3). The Floristic Quality Assessment (Table 2) showed a Total FQI of 47.4 and a Native FQI of 50.0. These FQI values are notably high, only exceeded in this study by those for the Ken-O-Sha Park and Stanaback Park floodplains. The Total Mean C, 3.7, for this site was the highest value of all seven sites inventoried. Six species have high-fidelity C-values of 8–10: green dragon (Arisaema dracontium), C-8; three sedges (Carex amphibola, C-8; C. laxiculmis, C-8; C. laxiflora, C-8); false rue anemone (Enemion biternatum), C-8; and swamp white oak (Quercus bicolor), C-8. The site is worthy of ongoing protection and conservation according to state-wide metrics for floristic quality (Table 1; Herman et al. 2001).
The floodplain forest along Plaster Creek in Paris Park is dominated by sugar maple (Acer saccharum), silver maple (Acer saccharinum), red maple (Acer rubrum), boxelder (Acer negundo), and white ash (Fraxinus americana); other tree species include black cherry (Prunus serotina), cottonwood (Populus deltoides), American elm (Ulmus americana), sycamore (Platanus occidentalis), and willows (Salix spp.). Poison-ivy (Toxicodendron radicans) abounds in the floodplain. A plethora of spring wildflowers is present, including common white trillium (Trillium grandiflorum), southern blue flag (Iris virginica), bloodroot (Sanguinaria canadensis), Canada anemone (Anemone canadensis), spring cress (Cardamine bulbosa), spring beauty (Claytonia virginica), yellow avens (Geum aleppicum), white avens (G. canadense), spring avens (G. vernum), may-apple (Podophyllum peltatum), swamp buttercup (Ranunculus abortivus), and rueanemone (Thalictrum thalictroides). Green dragon (Arisaema dracontium) and Jack-in-the-pulpit (A. triphyllum) are also remarkably frequent.
Wernlund Family Property (43.862°N, 85.585°W)
A total of 118 species, of which 78.8% are native, were recorded at this small privately owned parcel along Plaster Creek (Figure 3). The Floristic Quality Assessment (Table 2) showed a Total FQI of 30.4 and a Native FQI of 34.7; the Total Mean C was 2.8, equaling that of the Madison Avenue site as the lowest in our study. Four species have a C-value of 8–10: green dragon (Arisaema draconium), C-8; sedge (Carex tuckermanii), C-8; swamp white oak (Quercus bicolor), C-8; and bladdernut (Staphylea trifoliata), C-9 (Table 3).
Although non-native herbaceous species (21.2%) are widespread throughout this floodplain, this property boasts a diverse and mature canopy of native tree species. We noted that 16 of the 19 tree species are native, including sycamore (Platanus occidentalis), swamp white oak (Quercus bicolor), bur oak (Q. macrocarpa), black maple (Acer nigrum), black walnut (Juglans nigra), and hornbeam (Carpinus caroliniana). The small floodplain forest also supports five maple species: sugar maple (Acer saccharum), silver maple (A. saccharinum), red maple (A. rubrum), box elder (A. negundo), and the aforementioned black maple (Acer nigrum). Other native trees on the property include red ash (Fraxinus pennsylvanica), wild black cherry (Prunus serotina), chinquapin oak (Quercus muehlenbergii), basswood (Tilia americana), American elm (Ulmus americana), and shagbark hickory (Carya ovata).
Despite the floodplain forest understory bearing evidence of disturbance by several non-native species, many attractive native shrubs, wildflowers, and sedges persist in the floodplain. Notable native shrubs include bladdernut (Staphylea trifolia), buttonbush (Cephalanthus occidentalis), shrubby St. John’s-wort (Hypericum prolificum), spicebush (Lindera benzoin), and two species of native currants (Ribes americanum and R. cynosbati). The invasive and aggressive shrubs autumn olive (Elaeagnus umbellata) and multiflora rose (Rosa multiflora) are abundant as well.
Covenant Park (42° 53.520′N, 85° 34.980′W)
A total of 171 species, of which 74.9% are native, were recorded at this site along Plaster Creek, which had been a golf course until 2019 (Figure 4). The Floristic Quality Assessment (Table 2) showed a Total FQI of 37.9 and a Native FQI of 43.0. Eight species present at the site have a C-value of 8–10: green dragon (Arisaema dracontium), C-8; pawpaw (Asimina triloba), C-9; James’ sedge (Carex jamesii), C-8; hairy-fruited sedge (Carex trichocarpa), C-8; flowering dogwood (Cornus florida), C-8; false rue anemone (Enemion biternatum), C-8; swamp white oak (Quercus bicolor), C-8; and black snakeroot (Sanicula canadensis), C-8.
Of the 30 tree species documented, 25 are native. These include silver maple (Acer saccharinum), hackberry (Celtis occidentalis), three species of young ash trees (Fraxinus nigra, F. americana, and F. pennsylvanica), black walnut (Juglans nigra), black willow (Salix nigra), cottonwood (Populus deltoides), sycamore (Platanus occidentalis), swamp white oak (Quercus bicolor), and American elm (Ulmus americana). Pawpaw (Asimina triloba), shellbark hickory (Carya laciniosa), and bur oak (Quercus macrocarpa) are also well represented.
Covenant Park supports a diversity of high-quality native wildflowers, including an impressive display of Michigan’s spring flora. Among these are Canada anemone (Anemone canadensis), cut-leaved toothwort (Cardamine concatenata), spring beauty (Claytonia virginica), false rue-anemone (Enemion biternatum), both yellow trout lily (Erythronium americanum) and white troutlily (E. albidum), wood sandwort (Moehringia lateriflora), May-apple (Podophyllum peltatum), skunk cabbage (Symplocarpus foetidus), two species of meadow-rue (Thalictrum dasycarpum and T. dioicum), and three species of violets (Viola pubescens, V. sororia, and V. striata).
Several species in this remnant site are noteworthy, based on records from MICHIGAN FLORA ONLINE (2011). In early spring, several large patches of the less commonly seen white trout lily (Erythronium albidum) were encountered. Water dock (Rumex verticillatus) was found in abundance, despite having not been documented in the Grand Rapids area since Emma Cole’s collections in 1896 (Jenison, Ottawa County) and 1897 (Grand Rapids Township, Kent Co.). Our documentation of black snakeroot (Sanicula canadensis) represents a new county record for Kent County. Of the 19 sedge species (Carex spp.), three are especially notable: James’ sedge (Carex jamesii), a clump-forming species characteristic of rich moist forests that had been documented only twice in Kent County prior to the Emma Cole Grand Rapids Flora Project; Emory’s sedge (Carex emoryi), an uncommon sedge of riverbanks, had not been documented for Kent County since Emma Cole collected it in 1897 (at the Plaster Creek Crystal Springs site), and Carex davisii, a species only known locally from five river systems of southern Michigan (Clinton, Grand, Raisin, Rouge, and St. Joseph River systems) (MICHIGAN FLORA ONLINE 2011).
While it is remarkable that the intact remnant forested parcels have, collec-tively, retained a rather high FQA, we regret that much of the natural floodplain has been converted into fairways. Now that this large parcel is a public park, it would be desirable not only to preserve the remaining natural areas but to enhance their ecological quality and repair what has been damaged. We encourage Kentwood Parks and Recreation to consider restoring the presently unused fairways into more functional, biodiverse floodplain habitats, which would connect the isolated remnants into a much larger and more functional ecosystem.
Stanaback Park (42° 53.800’N, 85° 35.945’W)
A total of 215 species, of which 80.9% are native, were recorded in this large floodplain (Figure 5). The Floristic Quality Assessment (Table 2) showed a Total FQI of 48.4 and a Native FQI of 52.8, the second highest FQIs of all the sites studied––indicative of extraordinarily high quality, and a significant component of Michigan’s remaining native biodiversity––making this site especially worthy of protection (Table 1). The Total Mean C for this site (3.3) was intermediate among the seven sites inventoried (Table 1), yet several species have high-fidelity C-values of C-8–C-10: green dragon (Arisaema dracontium), C-8; pawpaw (Asimina triloba), C-9; sedge (Carex disperma), C-10; redbud (Cercis canadensis), C-8; panic grass (Dichanthelium lindheimeri), C-9; riverbank wildrye (Elymus riparius), C-8; and swamp white oak (Quercus bicolor), C-8.
The Plaster Creek floodplain in Stanaback Park has an open forest cover and supports 26 different native trees species, including many mature specimens. Among these are four species of maples (Acer saccharum, A. nigrum, A. saccharinum, A. negundo), pawpaw (Asimina triloba), hornbeam (Carpinus caroliniana), redbud (Cercis canadensis), two hawthorns (Crataegus succulenta and C. punctata), American beech (Fagus grandifolia), two species of young ash trees (Fraxinus nigra, F. pennsylvanica), black walnut (Juglans nigra), ironwood (Ostrya virginiana), sycamore (Platanus occidentalis), three species of oaks (Quercus bicolor, Q. macrocarpa, Q. muehlenbergii), sassafras (Sassafras albidum), and basswood (Tilia americana).
This site has perhaps the most impressive population of sycamore trees in the Grand Rapids area, a species typically found along rivers and streams in southern Michigan and states farther to the south. An especially noteworthy feature of this floodplain is that high in the treetops of one cluster of very large sycamores is a magnificent rookery of Great Blue Herons, consisting of about 20 nests (Figure 8).
Of the 8 shrub species present in the Stanaback Park floodplain, six are native, including buttonbush (Cephalanthus occidentalis), spicebush (Lindera benzoin), wild black currant (Ribes americanum) and three species of blackberry/raspberry (Rubus allegheniensis, R. occidentalis and R. pensilvanicus). Unhappily, two notoriously invasive non-native shrubs, autumn olive (Elaeagnus umbellata) and multiflora rose (Rosa multiflora), are widespread and common throughout the floodplain, the latter forming dense thickets in some noticeably disturbed areas.
The floodplain also supports a wealth of graminoids, including 20 species of grasses, 23 species of sedges, and three species of rushes. As a group, sedges make up an important component of Michigan’s native biodiversity, especially in wetland ecosystems like floodplains. All sedge species found growing at this site are native, and a few are of high-fidelity C-value. Carex disperma (C-10) has only been collected three times in Kent County, most recently in 1940; and Carex aquatilis, a wetland sedge (C-7), also had not been documented in Kent County since 1941 (MICHIGAN FLORA ONLINE 2011). Other distinctive sedges found at the site include Carex grayi, C. lupulina, C. gracilescens, and C. echinodes.
Several of the 20 species of grasses found in this floodplain have high C-values, as well. Panic grass (Dichanthelium lindheimeri) and riverbank wild-rye (Elymus riparius), both C-8, and wood reedgrass (Cinna arundinacea) and satin brome (Bromus nottowayanus), both C-7, were all documented in the floodplain. Over half of the species documented at the site are herbaceous. In fact, the floodplain supports an impressive 127 species of herbaceous plants, many with attractive flowers, and 46 species of graminoids. Numerous notable floodplain natives were found here, including green dragon (Arisaema dracontium), swamp milkweed (Asclepias incarnata), golden saxifrage (Chrysosplenium americanum)––which had not been collected along Plaster Creek since 1896, and not previously documented in Kent County since 1919 (MICHIGAN FLORA ONLINE 2011)––southern blue flag (Iris virginica), cardinal flower (Lobelia cardinalis), mermaid weed (Proserpinaca palustris), water dock (Rumex verticillatus), arum-leaved arrowhead (Sagittaria cuneata), water parsnip (Sium suave), common bur reed (Sparganium eurycarpum), skunk cabbage (Symplocarpus foetidus), and four species of violets (Viola cucullata, V. pubescens, V. sororia, V. striata).
Ken-O-Sha Park (42° 54.397’N, 85° 38.165’W)
A total of 214 species, of which 82.2% are native, were recorded along Plaster Creek at Ken-O-Sha Park (Figure 6). This site is on par with Stanaback Park as having the highest species richness. The Floristic Quality Assessment (Table 2) showed a Total FQI of 52.7 and a Native FQI of 58.4. These FQI values were the highest of the seven sites surveyed, exceeding the FQI threshold of greater than 50 (Table 1), indicating that the site exhibits extremely high conservation value and represents a significant component of Michigan’s native biodiversity and natural landscapes. While Ken-O-Sha boasts the highest FQIs of all sites, its Total Mean C of 3.6 is slightly lower than Paris Park’s Total Mean C of 3.7 and slightly higher than Stanaback Park’s Total Mean C of 3.3 (Table 2). Goforth et al. (2001) and Herman et al. (2001) do not find it unusual for sites with similar FQIs to have rather different Mean C-values. Of the 176 native species, more species with high-fidelity C-values (C-8–C-10) occur in this floodplain site than in any of the others. These include pawpaw (Asimina triloba), C-9; 5 species of sedges (Carex amphibola, C-8; C. laxiculmus, C-8; C. laxiflora, C-8; C. trichocarpa, C-8, C. woodii, C-8), redbud (Cercis canadensis), C-8; American cancer-root (Conopholis americana), C-10; riverbank wild-rye (Elymus riparius), C-8; swamp saxifrage (Micranthes pensylvanica), C-10; bluegrass (Poa alsodes), C-9; swamp white oak (Quercus bicolor), C-8; bladdernut (Staphylea trifolia); C-9; and rue-anemone (Thalictrum thalictroides), C-8.
The floodplain forest in this site supports 29 tree species. Black maple (Acer nigrum) is predominant, and boxelder (A. negundo), red maple (A. rubrum), silver maple (A. saccharinum) and sugar maple (A. saccharum) are common. Other trees that are present, but usually widely scattered and not in abundance, include bitternut hickory (Carya cordiformis), shagbark hickory (C. ovata) and pignut hickory (C. glabra), black ash (Fraxinus pennsylvanica), hackberry (Celtis occidentalis), black walnut (Juglans nigra), sycamore (Platanus occidentalis), swamp white oak (Quercus bicolor), white oak (Q. alba), red oak (Q. rubra), black cherry (Prunus serotina), cottonwood (Populus deltoides) and American elm (Ulmus americana). The understory layer is occupied by redbud (Cercis canadensis), hornbeam (Carpinus caroliniana), hop-hornbeam (Ostrya virginiana), spicebush (Lindera benzoin), and bladdernut (Staphylea trifoliata). Dotted hawthorn (Crataegus punctata) was sparse and scattered at this site, although just upstream at the Stanaback Park floodplain it is locally abundant, forming small groves.
A well-used asphalt walking trail begins at the parking area of the Ken-O-Sha Elementary School and parallels Plaster Creek along the length of Ken-O-Sha Park downstream, giving the general public access to experience the beauty of the rich deciduous beech-maple woods with frequent views into the lower floodplain. In spite of its heavy usage, the trail largely avoids the floodplain, thereby minimizing disturbance and helping to preserve the quality of the floodplain in this park. Yet the floodplain remains vulnerable to adventive and sometimes aggressive non-native species. This is one of only two localities where scattered plants of the ornamental shrub jetbead (Rhodotypus scandens) have become established. Likewise, the adventive Indian-strawberry (Potentilla indica) is known only from this site and the Madison Avenue Crossing site, which is just downstream. The recently spreading bitter cress (Cardamine impatiens) is common here; it has been noted by Voss and Reznicek (2012) as “A rapid invader of forest understories,” although it is currently known from only five counties in Michigan. Another adventive documented in only five Michigan counties, but abundant at this site in early spring, is lesser celandine (Ficaria verna).
Madison Avenue (42° 54.995’N, 85° 39.215’W)
A total of 153 species, of which 71.9% are native, were recorded at the small floodplain of Plaster Creek where it crosses Madison Avenue, along Plaster Creek Trail (Figure 7). This is the most urban floodplain among the seven we inventoried. The Floristic Quality Assessment (Table 1) showed a Total FQI of 34.6 and a Native FQI of 42.0 for this site. Its Total Mean C of 2.8 matched that of the small floodplain on the Wernlund Property, and is the lowest Total Mean C of the seven study sites; the Crystal Springs and Covenant Park sites have only a slightly higher Total Mean C of 2.9. A rather disturbed site, the Madison Avenue floodplain also ranked highest in percentage of non-natives (28.1%, 43 species). However, the north side of the creek––the least accessible portion of the floodplain––is far less disturbed and supports a very robust population of a rare graminoid, beak grass (Diarrhena obovata), with a high-fidelity C-value (C-9). This is also the site where our showy native redbud (Cercis canadensis), C-9, is best represented––a species Emma Cole (1901) noted as reaching its geographic northern limit along the Grand River within the Grand Rapids region. Another C-9 species, bladdernut (Staphylea trifolia), was also found here. Three additional high-fidelity C-value species encountered at this site include riverbank wild-rye (Elymus riparius), C-8; false rue anemone (Enemion biternatum), C-8; and swamp white oak (Quercus bicolor), C-8.
In sharp contrast to the high-value native species, garden escapes also appear in some of these natural-looking habitats. Wild-oats (Chasmanthium latifolium), an attractive and often cultivated grass species, was documented at the Madison Avenue crossing. This species is listed as native and Endangered in Michigan, but Voss and Reznicek (2012) state that while it is indeed native along the floodplain of the Galien River in Warren Woods, Berrien County, all Michigan records outside Berrien County are regarded as escapes from cultivation. Another escape from cultivation we encountered is a cluster of several small trees of the rutaceous Amur cork-tree (Phellodendron amurense). A third noteworthy adventive species at this site is lemon-balm (Melissa officinalis). Often cultivated for its aromatic oils, lemon-balm is seldom known as an escape, and our documentation is the first record for Kent County and sixth county documentation for the state (MICHIGAN FLORA ONLINE 2011). Black swallow-wort (Vincetoxicm nigrum), an aggressive weed that appears in many localities in Michigan, was collected here and in several other sites inventoried by the Emma Cole Grand Rapids Flora Project, but it has not yet appeared for Kent County on the MICHIGAN FLORA ONLINE (2011) website maps. We collected ivyleaved speedwell (Veronica hederifolia), which was not reported for Michigan until 1999, and but now known from seven sites in five counties (MICHIGAN FLORA ONLINE 2011); ours was the second collection from Kent County, the first having been collected along Plaster Creek in 2015 (Slaughter 1393 MICH) just one mile downstream from our site.
The Madison Avenue floodplain is yet another Plaster Creek site that directly connects our study with Emma Cole’s work. Cole (1901) mentions several plants from along Plaster Creek “at Madison Ave.,” although many of these are species of drier habitats and likely occurred along the high banks and bluffs of Plaster Creek. Ironically, while we report Veronica hederifolia as a relatively new adventive to Michigan, Emma Cole’s first encounter with Vernonica hederifolia was made on her four-month trip to Europe at Cave Hill, Belfast, Ireland, July 9, 1903 (Cole 51703 MICH), two years after the publication of her Grand Rapids Flora (Cole 1901).
Plaster Creek in the 1890s Compared to Today
A search of all Plaster Creek entries in Emma Cole’s (1901) Grand Rapids Flora was conducted, as well as a search of the MICHIGAN FLORA ONLINE (2011) database for Cole’s specimens collected along Plaster Creek, yielding 84 species. Although Cole did not always indicate specifically where along Plaster Creek her specimens were collected, it is reasonable to treat the whole of Plaster Creek as a single entity. However, a number of those species indicated as “Plaster Creek” were clearly not wetland plants, such as prairie smoke (Geum triflorum) and kitten-tail (Besseya bullii). Therefore, after removing Cole’s plants that clearly grow in dry sites, a total of 65 species were included in the Floristic Quality Assessment carried out for the 1890s material. Some of Cole’s records, such as showy lady-slipper (Cypripedium reginae), tawny cotton-grass (Eriophorum virginicum), and queen-of-the-prairie (Filipendula rubra) are likely to have occurred in fen-like seeps either immediately at the interface of floodplain with the base of ravines, or possibly seeps higher up on the banks of Plaster Creek. We have retained these species in our analysis because we also included such habitats in our inventories.
The 1890s Floristic Quality Assessment includes 98.5% (64 species) native species and only 1.5% (1 species) non-native. The Total FQI was 48.4 for this collection of species, and the Native FQI was 48.8; the Total Mean C was a robust 6.0. A total of 27 species had a high C-value of 8–10 (8 species with 10, 9 species with 9, and 10 species with 8) (Table 4).
The 40.6% of native species with high C-values from Cole’s list (26 of 64) is markedly higher than our present-day tally of only 7.9% (27 of 341) based on the combined Plaster Creek flora for the seven sites (Table 3). Furthermore, we were unable to locate 19 of the 27 species on Cole’s Plaster Creek list with C-values of 8–10. Although there had been no assigned C-values back in Emma Cole’s day, this comparison shows that many species that require undisturbed habitats are no longer present in our floodplain sites, despite Emma Cole’s work confirming that they once existed there. It is also notable that only 1.5% of the species on Cole’s list (1 of 65) were non-natives, which is markedly smaller than our sites, for which non-native species constitute 22.1% of the total number of plants documented (97 of 438).
While the loss of species reflected in these data is deeply regretful, it was encouraging to find several relatively rare species (locally and state-wide) persisting in these floodplains. For example, green dragon (Arisaema dracontium) had not been documented in Kent County since the late 1800s—twice by Emma Cole—yet we found it in four of our seven sites. We also found golden saxifrage (Chrysosplenium americanum), a species that had been documented only once, in 1939, since Emma Cole’s day, in the Stanaback floodplain. In addition, the state threatened Virginia bluebells (Mertensia virginica) is still thriving in multiple floodplain sites along Plaster Creek today. So, although several species appear to have been lost from our landscape over the past 120+ years, there remains a good amount of native Michigan biodiversity, even in urban greenspaces, that will benefit from sound preservation, conservation, and restoration efforts.
It must be noted that Emma Cole’s purpose in cataloging the 1275 species recorded in her Grand Rapids Flora (Cole 1901) was quite different from our effort to conduct full inventories of numerous high-value natural landscapes within the area covered by Cole. Her collections along Plaster Creek were not meant to fully capture the floristic composition of the floodplain. Yet, Cole’s documentation of 65 wetland species from the 1890s, though representing but a fraction of the actual Plaster Creek corridor flora, still provides a sense of the scope of change that has taken place across the Plaster Creek landscape since Emma Cole was botanizing this landscape.
Rare Plants (Past and Present)
Table 4 lists all species documented in the present study as well as those documented by Emma Cole in the 1890s from Plaster Creek that have a high level of fidelity to a narrow range of undisturbed ecological conditions, that is, those with C-values in the range 8–10, and also highlights those species that are listed by the Michigan Natural Features Inventory (MNFI 2009, updated March 2023) with a state status of Endangered (E), Threatened (T), or Special Concern (SC). Because of the sensitivity of any state-listed species that are threatened or endangered, we have withheld their locality data. In the following enumeration of these species, information regarding their broader occurrence in Michigan is drawn from the MNFI online database (available at https://mnfi.anr.msu.edu/species/plants).
Mertensia virginica (Virginia bluebells): Threatened. State-wide, this beautiful species is documented by 25 occurrences in 11 counties, including seven from Kent County (MNFI 2023). We found this plant growing in three of our floodplain sites, ranging from very sparse to robust populations (Figure 9). Emma Cole (1901) described it as “scarce” and as occurring in rich alluvial soil in scattered sites along the Grand River, Plaster Creek, and in woods within Byron Township. At the time of our study, this species was listed as Endangered (MNFI 2009), but as of March 20, 2023, the status was changed to Threatened (MNIF 2023).
Diarrhena obovata (beak grass): Formerly Threatened, now delisted. This species is documented as having 40 occurrences in 17 counties in Michigan, including three in Kent County (as of 2016) (MNFI 2009). At the time of this study, the species was listed as Threatened status (MNFI 2009), but has since been delisted by MNFI (2023). This species was not recorded by Cole (1901). We encountered Diarrhena obovata at three sites along the Plaster Creek floodplain, all with very robust populations; the size of the existing populations appears to be increasing. The robustness of populations we observed concurs with the assessment by MNFI (2023) to delist this species.
Filipendula rubra (Hill) B. L. Rob. (Queen-of-the-prairie): Threatened. A plant of wet prairies, fens and wet meadows with showy pink feathery panicles is documented as having 22 occurrences in six counties, but not in Kent County (MNFI 2023). Although we did not encounter this attractive species in our study, we have collected it at a wet meadow in Ada Township. Emma Cole (1901) referred to this species as Spiraea lobata Jacq. (Crow 2017). She considered it “rare,” citing only two populations, but she also noted that the species is often seen in cultivation. Voss and Reznick (2012) likewise note that the species is sometimes cultivated, regarding it native in Calhoun, Cass, Berrien and possibly Kent Counties, yet suggesting that even in those counties there may be populations that originated as escapes from cultivation.
Morus rubra L. (red mulberry): Threatened. A small tree of river bottoms, floodplains and swamps, red mulberry has been documented 42 times in 16 counties (MNFI 2023). We have not encountered this species despite numerous visits to habitats where it might be expected, including a visit to a particular location on the Grand River where it was documented by Emma Cole. Cole (1901) indicated that red mulberry populations were all in alluvial soil, but only “occasional” in occurrence. She cites “Plaster Creek” as well as localities along the Grand River as supporting this species.
Trillium nivale Riddell (snow trillium): Threatened. Snow trillium has been documented in ten localities within four counties in Michigan (MNFI 2023). Cole (1901) reported it as “rare; north bank of Plaster Creek” as well as at four other sites; four later collections document it from Kent County in MICHIGAN FLORA ONLINE (2011). Once known from the floodplain at Paris Township (now Ken-O-Sha Park, Grand Rapids), the species was last documented by two specimens collected by different botanists in 1939 (Reznicek, pers. comm.). Despite its historic presence along Plaster Creek, we did not encounter this species in our study. A story has circulated that, after having been shown this rare but beautiful plant on a class field trip, all students turned in their required plant collection projects––each having a specimen of Trillium nivale––resulting in the decimation of that population (as related by E. G. Voss in 1982 to Robert Bloye, pers. comm.).
Carex trichocarpa (hairy-fruited sedge): Special Concern. Occurrences of this rhizomatous, clonal sedge of riparian wetlands with characteristic reddish leaf bases have been documented from 21 localities in 9 counties, the most recent from Kent County in 1939 (MNFI 2009, 2023). In 2015, Warners spotted it along a creek at the edge of a golf course (Leisman, Van Staalduinen and Warners EC-15–319 CALVIN) that ultimately empties into Plaster Creek; he again discovered it at two of the floodplain sites of this study, Covenant Park and Ken-O-Sha Park. Interestingly, Cole’s (1901) recording of C. trichocarpa in her Grand Rapids Flora was based on a mis-determination of C. lupulina (Crow 2017).
Euonymus atropurpureus (wahoo or burning-bush): Special Concern. Occurrences of wahoo, an understory tree or tall shrub that is most easily recognized in fall by the presence of nodding pink capsules that dehisce to reveal seeds surrounded by a red fleshy aril is documented at 30 localities in 12 counties (MNFI 2009, 2023). Crow stumbled onto a single individual along Plaster Creek at the Crystal Springs site in the fall of 2022 (Crow 11181, CALVIN, MICH, MSC). According to MICHIGAN FLORA ONLINE (2011), the species had not been documented from Kent County since Emma Cole’s collections in 1896. The Emma Cole Grand Rapids Flora Project collected this species in 2017 in Ottawa County in one of the many ravines along the Grand River (Van Donselaar, Antuma, & Quakenbush EC-17-2200, CALVIN). Cole (1901) stated that this species was known from Ottawa County along the Grand River at West Bridge Street Ferry, at Boynton’s Landing, and at church picnic grounds in Jenison in Ottawa County. She also noted that in Kent County it occurred along the Grand River in Plainfield Village and in woods south of Reeds Lake. Cole reported that it was “formerly frequent, but it has been dug up and sold for medicinal purposes; now it is chiefly found in unfrequented places.”
Jeffersonia diphylla (L.) Pers. (twinleaf): Special Concern. Occurrences of twinleaf, an herb of rich woods and floodplains with a distinctive pair of stem leaves and petals that readily drop off soon after flowering (i.e., caducous), is documented from 34 occurrences in 16 counties (MNFI 2023). Emma Cole (1901) characterized twinleaf as “rare,” growing in rich moist woods; she added that in the 1870s “it grew along Plaster Creek, south of Hall St.” We did not encounter this distinctive species during our study of Plaster Creek floodplains but did collect it in 2019 in a rich woods in Byron Township, Kent County (Walt, Hartwig & Crow EC-19–4000, CALVIN, MICH).
Lithospermum latifolium (broad-leaved puccoon): Special Concern. Statewide occurrences of this species have been documented from 29 localities in 12 counties (MNFI 2009, 2023). It was encountered at only a single floodplain, Ken-O-Sha Park, in this study; however, we have otherwise documented it from three additional sites in Kent County (Stockdale et al., 2019; Warners et al. 2021). This species typically occurs in floodplain forests or rich ravines, where we have found it to be sparsely scattered. Although Cole (1901) did not specifically record this plant from Plaster Creek, she noted that it was “[F]requent on the edges of woods.”
Concluding Remarks
A close look at our seven sites reveals that even though all seven floodplain areas are within the same drainage basin, only 6.2% of the species we identified (27 of 438) were found in all seven floodplains (Table 3). By contrast, 39.7% (174 species) were found in only one site, a number that is remarkably similar to a comparison made among the inventories of nine forest remnants within a one square mile area in Lowell Township, Kent County (Warners et al. 2021). In that study, 37% of the total number of species were found in only one of the nine woodlots. These comparisons highlight that within the same habitat type and even within a very limited geographic zone, individual natural areas can harbor remarkably different species assemblages. More specifically, floodplain habitats within a single watershed can be dramatically dissimilar. Recognizing such diversity within habitat types highlights the importance of protecting and caring for every parcel of high-quality natural habitat that remains in human-dominated landscapes.
Although each site does harbor a valuable and unique assemblage of Michigan’s native floodplain flora, three of the seven sites we inventoried—the floodplains at Ken-O-Sha Park, Stanaback Park, and Paris Park—stand out. All three have high FQIs, and each represents a significant component of Michigan’s remaining native biodiversity (Tables 1 and 2). The floodplain at Stanaback Park is especially valuable given its impressive size for an urban natural area (nearly 22 hectares) and because it is relatively well-protected by surrounding woodlands and steep topography. We strongly urge the City of Kentwood Parks and Recreation Department to continue caring for this site in order to maintain the integrity of such a noteworthy example of southern Michigan floodplain habitat.
In sharp contrast, the Crystal Springs Plaster Creek site was the most degraded of the seven parcels. Of necessity, our inventory included only the portion of Plaster Creek floodplain that flows north from the Leisure Creek Drive bridge, within the condominium complex. Initially we intended to include the creek corridor south of the bridge to 68th St. SE in the study, but we found it to be an extremely narrow and eroded channel with adjacent vegetation consisting mostly of invasive trees and shrubs. That area subsequently became a restoration project of Calvin University’s Plaster Creek Stewards initiative in 2021–22 (Figure 10) (Calvin University 2023), funded by the Michigan Department of Environment, Great Lakes, and Energy. This restoration project will also include a major enhancement of the downstream section of floodplain that was included in this present study (Figure 2).
Many urban streams have become dangerously degraded over time due in part to the conversion of floodplain habitat to human-dominated landscapes. Damage done to these native floodplains has in turn hindered the important environmental services that healthy floodplains provide when floodwaters rise. Restoring streams back to healthier, more functional ecosystems will require bringing back floodplain habitats through restoration efforts. In the Plaster Creek watershed, locations such as Stanaback, Ken-O-Sha, and Paris Park still provide important environmental services. These parks are also important reference ecosystems that help restoration and conservation practitioners understand which species can persist in floodplain ecosystems within developed landscapes, thereby helping to inform successful future restoration efforts. Furthermore, these communities are important sources of native propagules for organizations like Plaster Creek Stewards who are working to increase the presence of functional and biodiverse floodplain habitat through ecological restoration efforts.
Acknowledgments
We would like to express our appreciation for the financial support provided at various stages of the project from the Hanes Fund, the Michigan Botanical Foundation, the Calvin University Alumni Association, and the Calvin University Science Division Summer Research Program. The Calvin University Herbarium (CALVIN) and the Michigan State University Herbarium (MSC) provided access to their collections to confirm identifications of challenging specimens and serve as repositories for voucher specimens. Critical specimens, including those documenting new county records and rare species, have also been deposited at the University of Michigan Herbarium (MICH). Dr. Anton A. Reznicek (MICH) kindly confirmed many of the critical Carex identifications and provided specimen information not readily available online. We are appreciative of the efforts of several people who participated in the field work of the Emma Cole Grand Rapids Flora project: Dorthea Leisman and Emily Van Staalduinen (2015), Alan Stockdale and Lydia Abma (2016), Zachary Hartwig (2019, 2021), Lucas Walker (2021), Natalie Vredevoogd (2021), William Hofmann, Hayden Janssen, and Martin Vanderschoot (2022). We are grateful to the editor-in-chief, Michael Huft, Anton Reznicek and an anonymous reviewer for their comments and suggestions which greatly improved the manuscript.
Literature Cited
Belknap, C. E. (1922). The yesterdays of Grand Rapids. The Dean-Hicks Co., Grand Rapids.
Belknap, C. E. (1926). Belknap writings and correspondence, Box 3, Folder: 7. Captain Charles Eugene Belknap Collection, Collection 300. Grand Rapids History Center, Grand Rapids, Michigan. Available at https://archive.grpl.org/repositories/4/archival_objects/32386. (Accessed on October 24, 2022).https://archive.grpl.org/repositories/4/archival_objects/32386
Calvin University. (2023). Plaster Creek Stewards. Available at https://calvin.edu/plaster-creek-stewards (Accessed on June 8, 2023).https://calvin.edu/plaster-creek-stewards
Cole, E. J. 1901. Grand Rapids flora: A catalog of flowering plants and ferns growing without cultivation in the vicinity of Grand Rapids, Michigan. V. Van Dort, Grand Rapids.
Crow, G. E. 2017. Emma Cole’s 1901 Grand Rapids Flora: Nomenclaturally updated and revised. The Great Lakes Botanist 56: 98–176.
Crow, G. E., D. P. Warners, H. R. Weesies, J. D. Walt, Z. E. Hartwig, C. R. Koehn. 2022. Botanical assessment of high-quality wetlands in the undeveloped Lowell Regional Greenspace, Kent County, Michigan. The Great Lakes Botanist 61: 2–34.
DeJong, R. (2017). Plaster Creek bacterial monitoring and source tracking project. (DEQ Tracking code #2015–0504). Available at https://calvin.edu/dA/c9c077f964/Plaster_Creek_Bacterial_Monitoring_and_Source_Tracking_Project.pdf?language_id=1 (Accessed on October 18, 2022).https://calvin.edu/dA/c9c077f964/Plaster_Creek_Bacterial_Monitoring_and_Source_Tracking_Project.pdf?language_id=1
DraftLogic. (2022). Google Maps Area Calculator Tool. Available at https://www.daftlogic.com/projects-google-maps-area-calculator-tool.htm (Accessed on October 19, 2022).https://www.daftlogic.com/projects-google-maps-area-calculator-tool.htm
FTC&H (Fishbeck, Thompson, Carr & Huber, Inc.). (2008). Plaster Creek watershed management plan. October 2008, Project No. G02408EC. Unpublished report prepared for Grand Valley Metropolitan Council. Available at https://calvin.edu/dotAsset/d1e3dd2c-85b2-45bd-9d10-4bcb6a9bf61a (Accessed on October 18, 2022).https://calvin.edu/dotAsset/d1e3dd2c-85b2-45bd-9d10-4bcb6a9bf61a
Freyman, W. A., L. A. Masters, and S. Packard. (2015). The universal floristic quality assessment (FQA): An online tool for ecological assessment and monitoring. Methods in Ecology and Evolution 7: 380–383.
Garfield, C. W. (1910). The people’s play grounds. Pp. 30–37 in Fortieth Annual Report of the Secretary of the State Horticultural Society of Michigan. Available at https://www.biodiversitylibrary.org/item/27416#page/40/mode/1up.https://www.biodiversitylibrary.org/item/27416#page/40/mode/1up
Goforth, R. R., D. Stagliano, J. Cohen, M. Penskar, Y. Lee, and J. Cooper. (2001). Biodiversity analysis of selected riparian ecosystems within a fragmented landscape. Michigan Natural Features Inventory Report No. 2001–06. Lansing, Michigan.
Grimsley, G. P. (1904). The gypsum of Michigan and the plaster industry. Geological Survey of Michigan, Vol. IX, Part II of Geological Survey of Michigan. The Board of Geological Survey, Lansing. Available at https://archive.org/details/gypsummichigana00grimgoog/page/n8/mode/2up.https://archive.org/details/gypsummichigana00grimgoog/page/n8/mode/2up
Hartley, M. J., and M. L. Hunter, Jr. (1998). A meta-analysis of forest cover, edge effects, and artificial nest predation rates. Conservation Biology 12: 465–469.
Herman, K. D., L. A. Masters, M. R. Penskar, A. A. Reznicek, G. S. Wilhelm, W. W. Brodovich, and K. P. Gardiner. (2001). Floristic quality assessment with wetland categories and examples of computer applications for the State of Michigan. Revised, second edition. Michigan Department of Natural Resources, Wildlife, Natural Heritage Program, Lansing, Michigan.
Hopkins, W. G. (1999). Introduction to plant physiology, 2nd edition. John Wiley & Sons, Inc., New York, N.Y.
Houghton, D. 1838. Report of the State Geologist (House Documents, 1838, No. 24, January 26 1838). Available at https://www.michigan.gov/-/media/Project/Websites/egle/Documents/Programs/OGMD/Catalog/03/Geological-Reports.pdf?rev=fa7dae80cbae44e69f20e2c67c3da06c (Accessed 16 June 2023).https://www.michigan.gov/-/media/Project/Websites/egle/Documents/Programs/OGMD/Catalog/03/Geological-Reports.pdf?rev=fa7dae80cbae44e69f20e2c67c3da06c
Houghton, D. 1839. Report of the state geologist in relation to the improvement of state salt springs. (Senate Documents, 1839, No. 1, 1 January 1839). Available at https://www.michigan.gov/-/media/Project/Websites/egle/Documents/Programs/OGMD/Catalog/03/Geological-Reports. pdf?rev=fa7dae80cbae44e69f20e2c67c3da06c (Accessed 16 June 2023).https://www.michigan.gov/-/media/Project/Websites/egle/Documents/Programs/OGMD/Catalog/03/Geological-Reports. pdf?rev=fa7dae80cbae44e69f20e2c67c3da06c
Lee, Y-E., and D. Warners. (2014). A summary of three antibiotic resistant bacterial studies of Plaster Creek by Calvin College Biology 250 students, spring, 2014. Available at https://calvin.edu/dA/8e2a112c7b/Three_Antibiotic_Resistant_Bacteria_Studies.pdf?language_id=1 (Accessed on October 18, 2022).https://calvin.edu/dA/8e2a112c7b/Three_Antibiotic_Resistant_Bacteria_Studies.pdf?language_id=1
Matthews, J. W., P. A. Tessene, S. M. Wiesbrook, and B. W. Zercher. (2005). Effect of area and isolation on species richness and indices of floristic quality in Illinois, USA wetlands. Wetlands 25: 607–615.
Matthews, J. W., G. Spyreas, and C. M. Long. (2015). A null model test of floristic quality assessment: Are plant species’ coefficients of conservatism valid? Ecological Indicators 52: 1–7.
MICHIGAN FLORA ONLINE. A. A. Reznicek, E. G. Voss and B. S. Walters. (2011). University of Michigan. Continually updated and available at http://michiganflora.net/home.aspx. (Frequently accessed 2017–2022).http://michiganflora.net/home.aspx
MNFI. (2009). Michigan Natural Features Inventory. Michigan’s rare plants. Available at https://mnfi.anr.msu.edu/species/plants. (Accessed on October 4, 2022).https://mnfi.anr.msu.edu/species/plants
Reznicek, A. A., M. R. Penskar, B. S. Walters, and B. S. Slaughter. (2014). Michigan floristic quality assessment database. Herbarium, University of Michigan, Ann Arbor, Michigan and Michigan Natural Features Inventory, Michigan State University, Lansing, Michigan. Available at http://michiganflora.net/home.aspx.http://michiganflora.net/home.aspx
Slaughter, B. S., A. A. Reznicek, M. R. Penskar, and B. S. Walters. (2015). Notes on the third edition of the floristic quality assessment of Michigan. Wetland Science and Practice 32: 28–32.
Spyreas, G. (2019). Floristic quality assessment: A critique, a defense, and a primer. Ecosphere 10: 1–18 (Article e02825). Available at https://esajournals.onlinelibrary.wiley.com/doi/10.1002/ecs2.2825https://esajournals.onlinelibrary.wiley.com/doi/10.1002/ecs2.2825
Stockdale, A. W., G. E. Crow, and D. P. Warners. (2019). Remnant natural areas in the greater Grand Rapids, Michigan region: Evaluating botanical change since the 1890s. The Great Lakes Botanist 58: 2–21.
Swink, E., and G. Wilhelm. (1979). Plants of the Chicago region, third edition. The Morton Arboretum, Lisle, Illinois.
Swink, E., and G. Wilhelm. (1994). Plants of the Chicago region, fourth edition. Indiana Academy of Science, Indianapolis.
Warners, D. P., G. E. Crow, J. D. Walt, C. R. Koehn, Z. E. Hartwig, and D. Clum. (2021). Botanical assessment of high-quality woodland parcels in the undeveloped Lowell Regional Greenspace, Kent County, Michigan. The Great Lakes Botanist 60: 110–148.
Voss, E. G., and A. A. Reznicek. (2012). Field manual of Michigan flora. University of Michigan Press, Ann Arbor.
Zipperer, W. C. (1993). Deforestation patterns and their effects on forest patches. Landscape Ecology 8: 177–184. doi.org/10.1007/BF00125349.doi.org/10.1007/BF00125349