Introduction
Most forests in northeastern Indiana are relatively small and isolated fragments of a formerly contiguous forest (Harman et al. 2019). Where forests do exist, they are often surrounded by artificial habitats, such as agricultural land or urban development. Edge effects on environmental gradients (e.g., light, moisture, temperature) and the limited size of core forest habitat results in changes in plant community structure and composition (Harman et al. 2019; Harper et al. 2005). These forest fragments provide essential landscape heterogeneity that provides habitat for arthropods, birds, and small mammals (Freemark and Merriam 1986; Myers and Marshall 2021; Nupp and Swihart 2000; Proesmans et al. 2019). The preservation of such isolated forest parcels has the potential to improve animal habitat and to protect rare plant communities (Rosenblatt et al. 1999; Fauth 2000; Diamond and Heinen 2016).
Floristic quality assessments (FQA) provide a systematic, repeatable approach to compare botanical communities within and between sites (Swink and Wilhelm 1994, Rothrock and Homoya 2005). Within FQA, there is a reliance on assigned C-values for each species encountered that facilitates the calculation of a floristic quality index (FQI) as an information statistic. While Swink and Wilhelm (1994) provided assigned C-values for species within the Chicago region, these values likely do not apply to locations outside of that region. For Indiana, C-values were subsequently assigned as only seven counties in Indiana are included in the Chicago region (Rothrock 2004, Rothrock and Homoya 2005). There are several criticisms of FQA, C-values, and FQI (Spyreas 2019), one of which is the subjectivity of assigned C-values. However, as an information statistic, there is aggregation of values within the calculation of Mean C-values and FQI, which will mitigate biases in certain species (Spyreas 2019). Additionally, there is inherent noise in the data related to differences in C-value lists and missing species from surveys (Rothrock and Homoya 2005). There are limitations to FQA and associated Mean C-value and FQI calculations, however, it is currently a usable tool for understanding community structure in relation to anthropogenic disturbance (Spyreas 2019, Werners et al. 2021).
Little Wabash River Nature Preserve (LWRNP) is a 14.3 ha property (of which approximately 13.0 ha is forested) within the Little River watershed that is located in Allen County in northeastern Indiana and is surrounded by agriculture, suburban development, and other forest fragments (Figure 1). LWRNP is situated in a geological valley feature created by the draining of Lake Maumee during the Wisconsin glaciation, known as the Maumee Megaflood (Fleming et al. 2018). Currently closed to the public, LWRNP is managed by ACRES Land Trust, which acquired the property in two units—the largest unit in 2004 (9.8 ha, all forested) and the smallest in 2015 (4.5 ha, 1.3 ha of which is an old field). In addition to ACRES Land Trust, LC Nature Park and Little River Wetlands Project are working to protect land within the Little River watershed. The objectives of this study were to characterize the plant community structure and composition at Little Wabash River Nature Preserve using systematic ecological surveys to associate community structure with environmental conditions and meandering floristic surveys to develop a comprehensive species list. Results from ecological and floristic surveys will be useful to ACRES Land Trust in making management decisions at the property and acquisition decisions in the region.
Materials and Methods
Site Description
The property is mostly forested, but a 1.3 ha open field area does exist on the western side (Figure 1). LWRNP is dominated (72% of the area) by Glynwood clay loam soil (6–12% slope, moderately well drained). The southeastern portion of the property (24% of the area) is Eel silt loam soil (0–2% slope, frequently flooded). A small portion (4% of area) of LWRNP is Glynwood silt loam soil (2–6% slope, moderately well drained). Within the forested area, there is a 0.6 ha pond with open water.
Ecological Surveys
Understory Surveys and Environmental Conditions
Understory plants were surveyed and ecological data recorded during three seasonal periods: May 13–18, July 13–21, and September 20–21 2019. To link plant community data to environmental conditions, we established seven transects, spaced 50 m apart, running southeast to northwest within LWRNP. Along each transect we established 1 m2 quadrats spaced 30 m apart; there were a total of 48 quadrats. As the transects were not of equal length, the number of quadrats per transect were not equal. We surveyed all quadrats during the May, July, and September surveys. Within each 1 m2 quadrat, we identified to species and counted individuals of all plants ≤ 2 m in height rooted in the quadrat. Species nomenclature throughout all surveys follows the Integrated Taxonomic Information System (ITIS, 2023). Voucher specimens were collected when species were encountered for the first time, whenever possible (i.e., we considered the population to be large enough and the species common enough), and deposited in the Purdue University Fort Wayne Department of Biological Sciences herbarium.
At the center of each quadrat, we measured photosynthetically active radiation (PAR) (μmol/m2/sec) 1 m above the soil surface with a six-sensor linear ceptometer (Spectrum Technologies, Aurora, Illinois), the percentage of volumetric soil moisture content with a time domain reflectometer with 12 cm sensor rods (Spectrum Technologies, Aurora, Illinois), litter depth (cm) with a meterstick, and the percentage of canopy cover with a spherical concave densiometer (Forestry Suppliers, Jackson, MS) using standard protocols (Lemon 1956). PAR light data was converted to percentage of available PAR by dividing the quadrat data by an unattended light sensor continuously logging 100% solar radiation in an open, unshaded portion of the property.
Midstory and Overstory Survey
Midstory plant surveys were conducted on August 10–11, 2019 within 25 m2 (5 m × 5 m) plots centered on each understory quadrat. All plants > 2 m in height and < 8 cm diameter at breast height (DBH, 1.37 m above the soil surface) were identified to species and stems were counted for each species.
Overstory plant surveys were conducted on August 10–11, 2019 within 500 m2 circular plots (12.62 m radius) centered on each understory quadrat. All trees (≥ 8 cm in DBH) were identified to species and basal area (m2/ha) was determined from 10-factor prism counts for each species. While not identified to species, we also counted standing dead trees. The relative dominance of each species was calculated as the basal area of the species, divided by the sum of the basal areas of all species multiplied by 100. The relative frequency of each species was calculated as the frequency of the species (the number of plots in which the species occurs divided by the total number of plots surveyed) divided by the sum of the frequencies of all species multiplied by 100. The relative density of each species was calculated as the number of individuals of the species divided by the total number of individuals of all species multiplied by 100. The importance values of each species were then calculated as the sum of the relative dominance, the relative frequency, and the relative density of that species, divided by three.
Floristic Surveys
The floristic surveys were conducted between April and October 2019 (18 visits, every 1–3 weeks during survey period, some visits in the same week) to ensure that all habitat areas within LWRNP were visited and that plant species not encountered during the ecological surveys would be cataloged. As the survey transects were spaced 50 m apart, there were clearly areas of the property that were not surveyed. The floristic surveys turned up additional species that were not encountered during the ecological surveys. Due to the stochastic nature of the floristic surveys, the location and environmental conditions were not recorded. However, voucher specimens were collected of species encountered for the first time, whenever possible, and deposited in the Purdue University Fort Wayne herbarium.
Analysis
Floristic Quality Assessment
For all species encountered in the ecological and floristic understory surveys, we used the coefficient of conservatism (C or C-value) assigned by Rothrock (2004) for Indiana for subsequent calculations. These C-values range from 0 to 10 with lower values associated with species that can tolerate disturbance and greater C-values associated with species that cannot tolerate disturbance. A floristics quality index (FQI) was calculated for the site based on C-values and provided a relative comparison value of the conservation importance as a remnant habitat. FQI was calculated as
where Mean C-value was the calculated mean value for all species C-values at LWRNP and N is the total of native species present in the site. We used Method 2 as described by Rothrock (2004) where non-native species have a C-value of zero.
Statistical Analysis
Species richness (the number of species present) was recorded and the species diversity, using Shannon’s index, was calculated for each understory quadrat and each midstory and overstory plot based on abundance (count of individuals). Shannon’s index is an information statistic used as a measure of entropy within an ecological community and of uncertainty (Hayek and Buzas 1997). We calculated Shannon’s index following Hayek and Buzas (1997) as
where pi is the proportion of the ith species (pi = ni/N, where ni is the abundance of the ith species and N is the total abundance). Total understory abundance (counts of all individuals), richness, and diversity were analyzed using mixed effect linear regression with each of the following environmental factors: percentage of available PAR, percentage of soil moisture, litter depth, and canopy cover as independent fixed factors and with survey month as a random effect. A Wald chi-square test was used to test the confidence in the influence of the fixed effects on the dependent variable. Nonmetric multidimensional scaling (NMDS) ordination was used to visualize understory plant community composition at LWRNP based on species stem counts using the metaMDS function in the vegan package with default options (Oksanen et al. 2022). Bray-Curtis dissimilarity was used as the distance measure within the NMDS ordination. Through the ‘autotransform=TRUE’ option, the data was transformed using a Wisconsin double standardization with square root function. Joint vectors were displayed to represent influence of environmental variables on the plot locations in species space. Environmental variables were midstory species richness and diversity, overstory species richness and diversity, overstory dead tree basal area, percentage of canopy cover, percentage of soil moisture, percentage of PAR, and litter depth. We used an R2 = 0.2 as an arbitrary threshold, omitting joint vectors from the NMDS plot that were below the threshold. Unweighted average linkage hierarchical clustering was used to identify separation in clusters within the NMDS plot. All analyses were conducted in R version 4.2.2 (R Core Team 2022).
Results
Ecological Surveys
Understory Survey
We encountered 118 understory species in 47 families (Table 1). Three quadrats had zero individuals and they were different locations during the survey period (one in May, two in September). Thirty-eight of the species occurred in only a single quadrat. Forty-one species occurred on only one sampling date, thirty on two dates, and forty-seven occurred on all three sampling dates.
Species encountered during the understory ecological surveys, the number of quadrats in which each occurred, and the mean number of individuals per quadrat (standard error in parentheses).
| Family | Scientific name | Plots | Count |
|---|---|---|---|
| Adoxaceae | Sambucus canadensis L. | 1 | 3.0 |
| Anacardiaceae | Toxicodendron radicans (L.) Kuntze | 16 | 7.3 (2.5) |
| Apiaceae | Cryptotaenia canadensis (L.) DC. | 1 | 9.0 |
| Apiaceae | Daucus carota L. | 11 | 32.0 (7.7) |
| Apiaceae | Erigenia bulbosa (Michx.) Nutt. | 1 | 7.0 |
| Apiaceae | Osmorhiza claytonii (Michx.) C.B. Clarke | 9 | 7.1 (1.8) |
| Apiaceae | Pastinaca sativa L. | 3 | 3.3 (1.9) |
| Apiaceae | Sanicula canadensis L. | 10 | 25.8 (8.5) |
| Apocynaceae | Apocynum cannabinum L. | 5 | 8.0 (4.3) |
| Apocynaceae | Asclepias syriaca L. | 2 | 2.0 (0.0) |
| Asparagaceae | Convallaria majalis L. | 1 | 330.0 |
| Asparagaceae | Maianthemum racemosum (L.) Link | 1 | 1.0 |
| Asparagaceae | Polygonatum biflorum (Walter) Elliott | 2 | 2.0 (0.7) |
| Aspleniaceae | Asplenium platyneuron (L.) Britton, Sterns & Poggenb. | 1 | 3.0 |
| Asteraceae | Ageratina altissima (L.) R.M. King & H. Rob | 7 | 11.0 (2.6) |
| Asteraceae | Ambrosia artemisiifolia L. | 4 | 3.0 (0.8) |
| Asteraceae | Arctium minus (Hill) Bernh. | 2 | 2.5 (1.1) |
| Asteraceae | Cirsium arvense (L.) Scop. | 2 | 1.0 (0.0) |
| Asteraceae | Erigeron annuus (L.) Pers. | 7 | 13.4 (6.1) |
| Asteraceae | Euthamia graminifolia (L.) Nutt. | 1 | 2.0 |
| Asteraceae | Leucanthemum vulgare Lam. | 9 | 2.8 (0.6) |
| Asteraceae | Packera glabella (Poir) C. Jeffrey | 1 | 1.0 |
| Asteraceae | Solidago altissima L. | 5 | 9.0 (2.2) |
| Asteraceae | Solidago canadensis L. var. hargeri Fernald | 8 | 28.1 (9.3) |
| Asteraceae | Solidago sp. L. | 4 | 17.5 (8.3) |
| Asteraceae | Symphyotrichum lanceolatum (Willd.) G.L. Nesom | 9 | 20.1 (6.4) |
| Asteraceae | Symphyotrichum shortii (Lindl.) G.L. Nesom | 1 | 5.0 |
| Asteraceae | Taraxacum officinale F.H. Wigg. | 7 | 3.4 (1.1) |
| Asteraceae | Vernonia gigantea (Walter) Trel. | 2 | 7.0 (4.2) |
| Balsaminaceae | Impatiens capensis Meerb. | 7 | 10.6 (2.8) |
| Brassiaceae | Cardamine douglassii Britton | 1 | 1.0 |
| Brassicaceae | Alliaria petiolata (M. Bieb.) Cavara & Grande | 13 | 16.6 (5.3) |
| Brassicaceae | Cardamine concatenata (Michx.) Sw. | 2 | 8.0 (2.8) |
| Brassicaceae | Lepidium campestre (L.) W.T. Aiton | 2 | 9.5 (1.8) |
| Cannabaceae | Celtis occidentalis L. | 10 | 1.8 (0.3) |
| Caprifoliaceae | Lonicera maackii (Rupr.) Herder | 21 | 5.5 (1.2) |
| Caryophyllaceae | Cerastium fontanum Baumg. | 1 | 4.0 |
| Caryophyllaceae | Stellaria media (L.) Vill. | 1 | 4.0 |
| Celastraceae | Euonymus atropurpureus Jacq. | 2 | 1.5 (0.4) |
| Cornaceae | Cornus drummondii C.A. Mey | 3 | 2.0 (0.5) |
| Cornaceae | Cornus racemosa Lam. | 3 | 2.0 (0.5) |
| Cyperaceae | Carex granularis Muhl. ex Willd. | 1 | 1.0 |
| Cyperaceae | Carex jamesii Schwein. | 13 | 7.5 (2.4) |
| Cyperaceae | Carex normalis Mack. | 1 | 2.0 |
| Cyperaceae | Carex stipata Muhl. ex Willd. | 7 | 6.7 (2.2) |
| Cyperaceae | Carex vulpinoidea Michx. | 1 | 7.0 |
| Cyperaceae | Scirpus atrovirens Willd. | 1 | 1.0 |
| Elaeagnaceae | Elaeagnus umbellata Thunb. | 8 | 6.0 (1.6) |
| Fabaceae | Cercis canadensis L. | 3 | 9.3 (4.8) |
| Fabaceae | Medicago sativa L. | 1 | 1.0 |
| Fabaceae | Trifolium pratense L. | 9 | 12.7 (3.4) |
| Fabaceae | Trifolium repens L. | 5 | 9.4 (2.3) |
| Fagaceae | Quercus alba L. | 1 | 2.0 |
| Fagaceae | Quercus bicolor Willd. | 1 | 4.0 |
| Fagaceae | Quercus rubra L. | 2 | 1.0 (0.0) |
| Geraniaceae | Geranium maculatum L. | 1 | 28.0 |
| Grossulariaceae | Ribes cynosbati L. | 2 | 1.0 (0.0) |
| Hydrophyllaceae | Hydrophyllum appendiculatum Michx. | 1 | 1.0 |
| Hydrophyllaceae | Hydrophyllum macrophyllum Nutt. | 1 | 2.0 |
| Juglandaceae | Carya cordiformis (Wangenh.) K. Koch | 2 | 3.0 (0.0) |
| Juncaceae | Juncus tenuis Willd. | 4 | 4.5 (1.3) |
| Lamiaceae | Blephilia hirsuta (Pursh) Benth. | 4 | 3.8 (1.1) |
| Lamiaceae | Glechoma hederacea L. | 3 | 3.0 (0.9) |
| Lamiaceae | Prunella vulgaris L. | 3 | 8.3 (2.6) |
| Liliaceae | Erythronium americanum Ker Gawl. | 3 | 12.7 (2.6) |
| Limnanthaceae | Floerkea proserpinacoides Willd. | 6 | 13.0 (3.4) |
| Menispermaceae | Menispermum canadense L. | 1 | 8.0 |
| Montiaceae | Claytonia virginica L. | 1 | 4.0 |
| Oleaceae | Fraxinus pennsylvanica Marshall | 27 | 15.9 (5.0) |
| Oleaceae | Fraxinus quadrangulata Michx. | 2 | 12.5 (5.3) |
| Onagraceae | Circaea lutetina L. ssp. canadensis (L.) Asch. & Magnus | 14 | 12.2 (3.0) |
| Oxalidaceae | Oxalis dillenii Jacq. | 7 | 5.7 (2.2) |
| Phrymaceae | Phryma leptostachya L. | 1 | 1.0 |
| Pinaceae | Pinus strobus L. | 1 | 3.0 |
| Plantaginaceae | Plantago lanceolata L. | 10 | 36.9 (8.5) |
| Plantaginaceae | Plantago rugelii Decne. | 3 | 1.7 (0.3) |
| Poaceae | Agrostis gigantea Roth | 9 | 38.8 (6.8) |
| Poaceae | Bromus pubescens Muhl. ex Willd. | 1 | 2.0 |
| Poaceae | Dactylis glomerata L. | 2 | 10.0 (0.0) |
| Poaceae | Dichanthelium boscii (Poir.) Gould & C.A. Clark | 5 | 2.4 (0.7) |
| Poaceae | Dichanthelium linearifolium (Scribn. ex Nash) Gould | 7 | 9.3 (1.8) |
| Poaceae | Elymus hystrix L. | 1 | 7.0 |
| Poaceae | Glyceria striata (Lam.) Hitchc. | 14 | 30.3 (5.9) |
| Poaceae | Phleum pratense L. | 8 | 107.1 (22.1) |
| Poaceae | Poa pratensis L. ssp. pratensis | 2 | 22.5 (0.4) |
| Poaceae | Poa sylvestris A. Gray | 3 | 15.3 (10.1) |
| Polygonaceae | Persicaria virginiana (L.) Gaertn | 22 | 29.3 (5.4) |
| Ranunculaceae | Actaea pachypoda Elliott | 1 | 10.0 |
| Ranunculaceae | Anemone canadensis L. | 1 | 4.0 |
| Ranunculaceae | Ranunculus abortivus L. | 1 | 2.0 |
| Ranunculaceae | Ranunculus recurvatus Poir. | 5 | 2.2 (0.7) |
| Rosaceae | Duchesnea indica (Andrews) Focke var. indica | 2 | 6.5 (3.2) |
| Rosaceae | Fragaria vesca L. | 5 | 6.0 (1.9) |
| Rosaceae | Fragaria vesca L. subps. vesca | 3 | 1.7 (0.5) |
| Rosaceae | Geum canadense Jacq. | 16 | 7.5 (1.3) |
| Rosaceae | Geum sp. L. | 8 | 11.6 (4.0) |
| Rosaceae | Geum vernum (Raf.) Torr. & A. Gray | 13 | 2.5 (0.3) |
| Rosaceae | Prunus serotina Ehrh. | 6 | 1.5 (0.2) |
| Rosaceae | Rosa multiflora Thunb. | 7 | 5.9 (1.6) |
| Rosaceae | Rubus occidentalis L. | 6 | 5.7 (1.3) |
| Rubiaceae | Galium aparine L. | 18 | 5.4 (0.9) |
| Rubiaceae | Galium asprellum Michx. | 1 | 1.0 |
| Rubiaceae | Galium circaezans Michx. | 1 | 1.0 |
| Rubiaceae | Galium concinnum Torr. & A. Gray | 2 | 6.5 (3.2) |
| Rubiaceae | Galium triflorum Michx. | 7 | 3.1 (0.8) |
| Sapindaceae | Acer saccharum Marshall | 6 | 2.3 (1.0) |
| Sapindaceae | Aesculus glabra Willd. | 1 | 2.0 |
| Smilaceae | Smilax sp. L. | 1 | 2.0 |
| Smilaceae | Smilax tamnoides L. | 1 | 2.0 |
| Solanaceae | Solanum carolinense L. | 3 | 1.7 (0.5) |
| Ulmaceae | Ulmus americana L. | 10 | 2.5 (0.7) |
| Urticaceae | Boehmeria cylindrica (L.) Sw. | 1 | 1.0 |
| Urticaceae | Laportea canadensis (L.) Benth. | 1 | 37.0 |
| Urticaceae | Pilea pumila (L.) A. Gray | 7 | 16.0 (7.0) |
| Violaceae | Viola sororia Willd. | 8 | 32.1 (6.6) |
| Violaceae | Viola striata Aiton | 3 | 18.0 (13.1) |
| Vitaceae | Parthenocissus quinquefolia (L.) Planch. | 29 | 14.3 (2.4) |
| Vitaceae | Vitis vulpina L. | 8 | 1.5 (0.3) |
With month as a random effect, understory abundance (X2 = 38.18, p < 0.001), richness (X2 = 43.63, p < 0.001), and diversity (X2 = 14.64, p < 0.001) were positively related to the percentage of available PAR (Figure 2). Similarly, abundance (X2 = 14.37, p < 0.001) was positively related to the percentage of soil moisture, however, richness and diversity were not related to soil moisture (X2 = 2.68, p = 0.102; X2 = 0.05, p = 0.821; respectively; Figure 2). Litter depth did not have a significant influence on abundance (X2 = 1.48, p = 0.223), richness (X2 = 2.68, p = 0.102), or diversity (X2 = 3.26, p = 0.071) (Figure 2). As would be expected, canopy cover had an inverse influence on abundance (X2 = 145.16, p < 0.001), richness (X2 = 107.13, p < 0.001), and diversity (X2 = 27.13, p < 0.001) compared to available PAR (Figure 2).
The relationships between understory abundance, richness, and diversity and environmental variables (the percentage of avail- able photosynthetically active radiation [PAR], the percentage of volumetric soil moisture content, litter depth, and the percentage of canopy cover). Only significant relationships are represented by a line. The regression analysis included the survey month as a random effect.
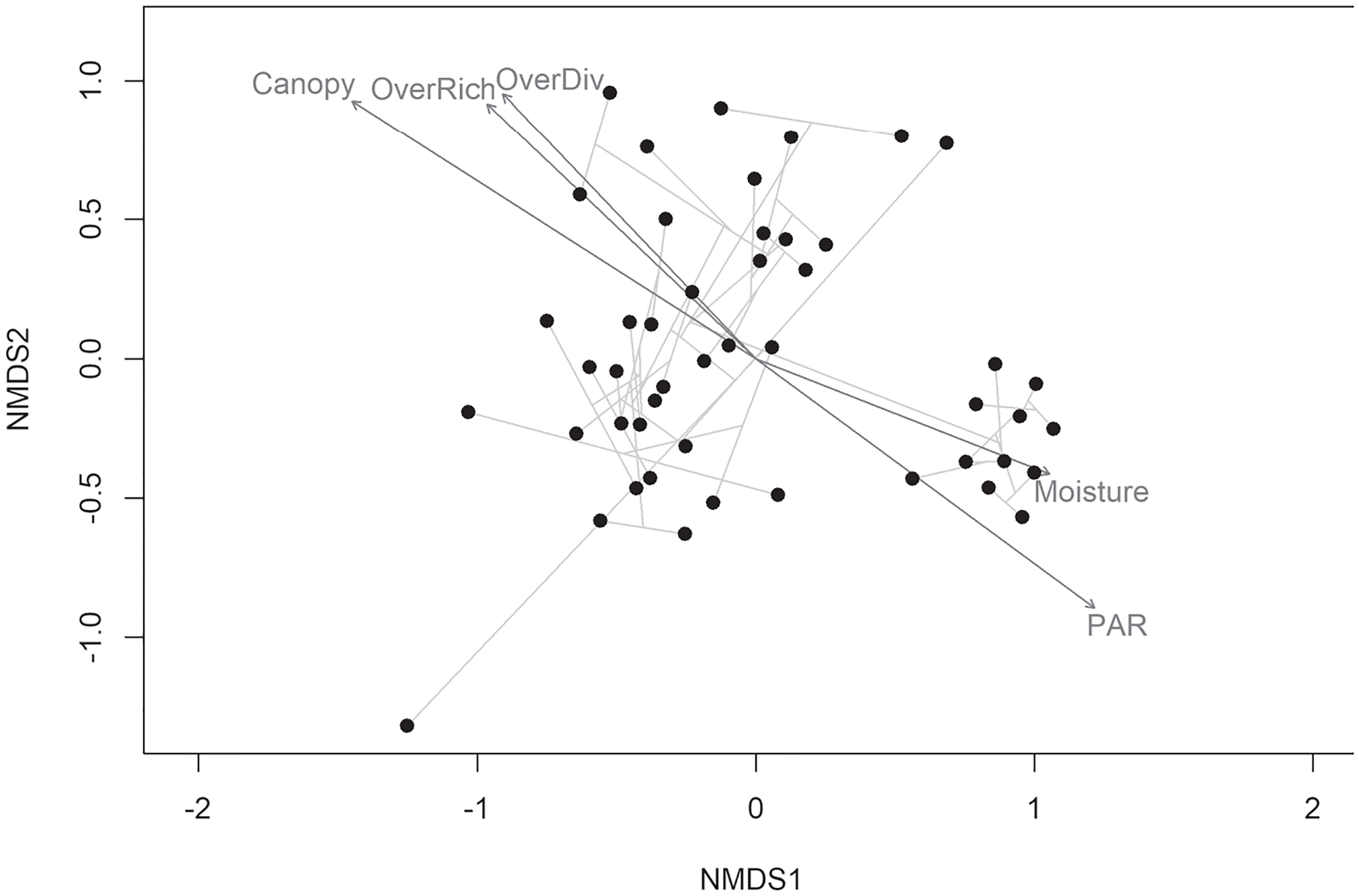
NMDS ordination was used to visualize the understory plant community at LWRNP (Figure 3). A small cluster of eleven plots were separate from the other plots within the NMDS. These included plots that occurred in the old field on the western side of LWRNP and along the transects adjacent to the old field. The separation of this cluster in the NMDS was positively influenced by soil moisture and available PAR in those plots. Conversely, this cluster was negatively influenced by canopy cover, overstory richness, and overstory diversity (Figure 3).
Nonmetric multidimensional scaling (NMDS) ordination of understory species based on stem counts within Little Wabash River Nature Preserve. Joint vectors represent relationships with environmental variables with an R2 > 0.2; Canopy is percentage of overstory canopy cover, Moisture is the volumetric percentage of soil moisture content, PAR is the percentage of available photosynthetically active radiation, OverRich is overstory species richness, and OverDiv is the Shannon diversity index of overstory species. The light gray lines connecting points represent unweighted average linkage hierarchical clustering.
Midstory Survey
We encountered 23 midstory species in 12 families (Table 2). Nine plots contained no midstory individuals. The non-native Lonicera maackii (Ruper.) Herder was by far the most frequently occurring (i.e., occurred in the greatest number of plots) and most abundant (i.e., with the greatest number of individuals per plot) midstory species encountered (Table 2). Other non-native species were observed in the midstory plots (Lonicera tatarica L., Elaeagnus umbellata Thunb., and Rosa multiflora Thunb.), but they were much less common than L. maackii. Fraxinus pennsylvanica Marshall was the most frequent native species (Table 2).
Species encountered in the midstory ecological surveys, the number of plots in which each occurred, and the mean number of individuals per plot (standard error in parentheses).
| Family | Species name | Plots | Count |
|---|---|---|---|
| Adoxaceae | Sambucus canadensis L. | 2 | 3.0 (2.0) |
| Cannabaceae | Celtis occidentalis L. | 8 | 1.8 (0.4) |
| Caprifoliaceae | Lonicera maackii (Ruper.) Herder | 30 | 11.6 (1.4) |
| Caprifoliaceae | Lonicera tatarica L. | 3 | 5.0 (1.9) |
| Cornaceae | Cornus drummondii C.A. Mey. | 10 | 3.9 (1.2) |
| Cornaceae | Cornus racemosa Lam. | 6 | 3.3 (0.9) |
| Elaeagnaceae | Elaeagnus umbellata Thunb. | 10 | 3.3 (1.0) |
| Fagaceae | Quercus bicolor Willd. | 5 | 1.2 (0.2) |
| Fagaceae | Quercus coccinea Münchh. | 1 | 1.0 |
| Fagaceae | Quercus rubra L. | 2 | 1.0 |
| Juglandaceae | Carya cordiformis (Wangenh.) K. Koch | 5 | 1.2 (0.2) |
| Juglandaceae | Carya ovata (Mill.) K. Koch | 3 | 1.0 |
| Juglandaceae | Juglans nigra L. | 4 | 1.0 |
| Oleaceae | Fraxinus pennsylvanica Marshall | 18 | 4.4 (0.6) |
| Oleaceae | Fraxinus quadrangulata Michx. | 3 | 3.7 (1.2) |
| Rosaceae | Crataegus sp. L. | 1 | 7.0 |
| Rosaceae | Prunus serotina Ehrh. | 1 | 1.0 |
| Rosaceae | Prunus virginiana L. | 1 | 8.0 |
| Rosaceae | Rosa multiflora Thunb. | 1 | 2.0 |
| Sapindaceae | Aesculus glabra Willd. | 2 | 1.5 (0.4) |
| Sapindaceae | Acer saccharum Marshall | 4 | 1.5 (0.4) |
| Ulmaceae | Ulmus americana L. | 1 | 1.0 |
| Ulmaceae | Ulmus rubra Muhl. | 1 | 2.0 |
Overstory Survey
We encountered 32 overstory species in 16 families (Table 3). Eleven plots had no overstory individuals within the 500 m2 circular boundary. Juglans nigra L. was the most frequently occurring and most dominant species, resulting in being the top-ranked species by importance value (Table 3). The spatial arrangement of J. nigra we observed in the forest (not quantified) suggests it was planted by previous land owners and may not represent natural recruitment of the species—regular spacing, stems of equal size. Standing dead trees, which we treated as a single species, had the third highest importance value, outranking Acer saccharum Marshall due to frequency (Table 3).
Species encountered during the overstory ecological surveys and the frequency (number of plots), density (mean number of stems per plot) (standard error in parentheses), dominance (basal area in m2/ha), and importance value (IV) of each.
| Family | Species name | Frequency | Density | Dominance | IV |
|---|---|---|---|---|---|
| Altingaceae | Liquidambar styraciflua L. | 5 | 2.7 (0.8) | 0.70 | 3.43 |
| Betulaceae | Betula papyrifera Marshall | 1 | 1.0 | 0.05 | 0.76 |
| Betulaceae | Carpinus caroliniana Walter | 1 | 1.0 | 0.05 | 0.76 |
| Betulaceae | Ostrya virginiana (Mill.) K. Koch | 1 | 1.0 | 0.05 | 0.76 |
| Cannabaceae | Celtis occidentalis L. | 6 | 1.2 (0.3) | 0.37 | 2.44 |
| Cornaceae | Cornus drummondii C.A. Mey. | 2 | 1.0 | 0.10 | 1.06 |
| Cornaceae | Cornus racemosa Lam. | 3 | 2.7 (1.4) | 0.42 | 2.56 |
| Cupressaceae | Juniperus virginiana L. | 5 | 5.8 (2.2) | 1.51 | 6.12 |
| Cupressaceae | Taxodium distichum (L.) Rich. | 1 | 4.0 | 0.21 | 2.39 |
| Ebenaceae | Diospyros virginiana L. | 1 | 3.0 | 0.16 | 1.85 |
| Fagaceae | Quercus alba L. | 1 | 1.0 | 0.05 | 0.76 |
| Fagaceae | Quercus bicolor Willd. | 15 | 4.3 (0.6) | 3.34 | 10.46 |
| Fagaceae | Quercus coccinea Münchh. | 1 | 1.0 | 0.05 | 0.76 |
| Fagaceae | Quercus muehlenbergii Engelm. | 2 | 1.0 | 0.10 | 1.06 |
| Fagaceae | Quercus rubra L. | 10 | 1.6 (0.3) | 0.83 | 4.22 |
| Juglandaceae | Carya cordiformis (Wangenh.) K. Koch | 4 | 2.9 (0.9) | 0.60 | 3.15 |
| Juglandaceae | Carya ovata (Mill.) K. Koch | 6 | 1.4 (0.3) | 0.44 | 2.65 |
| Juglandaceae | Juglans nigra L. | 20 | 4.0 (0.7) | 4.20 | 12.76 |
| Magnoliaceae | Liriodendron tulipifera L. | 4 | 1.9 (0.9) | 0.39 | 2.36 |
| Oleaceae | Fraxinus pennsylvanica Marshall | 7 | 1.3 (0.2) | 0.47 | 2.87 |
| Oleaceae | Fraxinus quadrangulata Michx. | 2 | 2.0 | 0.21 | 1.69 |
| Pinaceae | Picea glauca (Moench) Voss | 1 | 3.0 | 0.16 | 1.85 |
| Pinaceae | Pinus strobus L. | 2 | 1.5 (0.4) | 0.16 | 1.38 |
| Rosaceae | Crataegus sp. L. | 1 | 3.0 | 0.16 | 1.85 |
| Rosaceae | Prunus serotina Ehrh. | 10 | 3.6 (1.1) | 1.85 | 6.73 |
| Salicaeae | Populus deltoides W. Bartram ex Marshall | 2 | 3.0 (0.7) | 0.31 | 2.30 |
| Sapindaceae | Acer negundo L. | 1 | 1.0 | 0.05 | 0.76 |
| Sapindaceae | Acer saccharinum L. | 1 | 1.0 | 0.05 | 0.76 |
| Sapindaceae | Acer saccharum Marshall | 10 | 3.6 (0.8) | 1.85 | 6.73 |
| Sapindaceae | Aesculus glabra Willd. | 1 | 2.0 | 0.10 | 1.30 |
| Ulmaceae | Ulmus americana L. | 7 | 1.9 (0.5) | 0.70 | 3.50 |
| Ulmaceae | Ulmus rubra Muhl. | 1 | 1.0 | 0.05 | 0.76 |
| Dead trees | 17 | 1.9 (0.3) | 1.70 | 7.25 |
Floristic Surveys
We conducted floristic surveys 18 times during the survey period, none of which individually covered the entire property. During the floristic surveys, we encountered an additional 137 species unique to the floristic survey – we did encounter 99 species shared with the ecological surveys (see Appendix 1). Several of the species found only during the floristic survey were of note, including eight species of Cyperaceae (sedges), two of Ophioglossaceae (adder’s-tongue ferns), and three of Orchidaceae (orchids). While these were not necessarily rare, the were found because of the numerous visits with the floristic survey.
There were six species we could only identify to genus (Appendix 1). In each case, the individuals encountered were lacking key diagnostic characteristics and we were unable to confidently identify the species. It is possible that these specimens were actually the same as other species identified in the genera. Omitting these six species only identified to genus, we encountered 251 species in the three strata (Appendix 1).
Only two species encountered at LWRNP had high C-values (i.e., intolerant of disturbance) –Spiranthes magnicamporum Sheviak (found in a subsequent visit in 2022) and Taxodium distichum (L.) Rich. var. distichum (Cupressaceae). Most species had a C-value of 6 or less (93.3% of those with assigned C-values). Approximately 17.3% of species did not have an assigned C-value due to being non-native species. The mean C-value for LWRNP was 2.89; based on 209 native species, LWRNP had an FQI of 41.56.
Discussion
As most forests in northeast Indiana are relegated to disjunct fragments of a once-continuous forest, all well-established closed canopy forests represent important habitat for plants and animals in the region (Harman et al. 2019). Although LWRNP has a relatively open canopy (mean canopy cover = 71.5% across sampling dates in July and September), it is still an important part of the forest matrix in the region, and although it has clear evidence of past human manipulation—e.g., winter aerial images display clear fence-line plantings of conifers, observed plantation patterns of Juglans nigra, occurrences of Taxodium distichum in the understory and overstory well beyond the northern range in the Midwest (Wilhite and Toliver 1990)—species of interest were nevertheless encountered.
We found three orchid species (Orchidaceae), two of which, Liparis liliifolia (L.) Rich. ex Ker Gawl. and Spiranthes lacera (Raf.) Raf. var. gracilis (Bigelow) Luer, have limited occurrence records in northeastern Indiana. These two orchid species, in addition to Spiranthes cernua (L.) Rich., have relatively low C-value (3), which indicates species that provide little or no confidence that its habitat signifies remnant conditions (Rothrock 2004). This suggests that they are adapted to habitats that are at least somewhat disturbed. Since they were found in a relatively disturbed portion of the property, management in that area to reduce overstory and midstory canopy, as well as to provide regular disturbance, will likely promote success in L. liliifolia and S. lacera var. gracilis, especially since closed canopy mature forest is not suitable habitat for these species (Morris 1989, Mattrick 2004). Spiranthes magnicamporum and Spiranthes ovalis, were observed during a subsequent site visit in 2022 as we were confirming the identification of S. cernua. We included S. magnicamporum and S. ovalis in Appendix 1 with the indication that they were observed outside of our original floristic survey dates.
Spiranthes magnicamporum (added in the subsequent visit in 2022) and Taxodium distichum were the only two species encountered at LWRNP that had a C-value of 10, the latter of which, as noted above, is outside its natural range at LWRNP. Only 6.7% of the species encountered in the ecological and floristic surveys had a C-value > 6; C-values of 6 and below are associated with species able to tolerate significant or moderate disturbance (Rothrock 2004). Wilhelm et al. (2003) suggested that habitats with Mean C-values of 2 or less are typically old fields and highly degraded sites. Additionally, habitats with Mean C-values of 5 or more would be sites characteristic of a pre-European settlement plant community (Rothrock 2004). The Mean C-value at LWRNP was 2.87, which further supports our interpretation that human influence has played a significant role at the site. This low Mean C-value suggests that there has been significant disturbance to the site, although it may not be fully degraded. FQI values are collinear with species richness (i.e., FQI values align with species richness and are influenced by similar environmental factors), and Rooney and Rogers (2002) suggested using Mean C-value as a modified FQI value, which may be less influenced by the same environmental factors as species richness. Our FQI value at LWRNP (41.56) would suggest it is an exceptional site floristically, even with the extensive human influence of disturbance. This FQI value may be an over-estimate of the floristic quality, however, the Mean C-value (2.87) may be an under-estimate of the floristic quality. By comparison, Fogwell Forest Nature Preserve (same county, 6.5 km away) has a Mean C-value of 3.60 and an FQI of 55.4, which has a plant community indicative of limited disturbance (Rothrock and Homoya 2005, Arvola et al. 2014). Rothrock (1997) noted the absence of non-native species were limited to the ecotone and old field and not in the core of the forest.
Even more evidence of the human influence at LWRNP was found in the midstory. Although there were only four non-native species in the midstory, they made up 65% of the total number of midstory individuals. The remaining 45% of midstory individuals belonged to 19 native species. The non-native Lonicera maackii accounted for 56% of all midstory individuals.
Long-term human impact on the plant community is evident in the overstory. Some of the overstory species with the five highest importance values were expected, while others were not. The overstory species with the highest importance value was Juglans nigra, which does not commonly dominate forests in northeastern Indiana, and Eyre (1980) does not define a Black Walnut forest type. The economic value of J. nigra likely led to the mass planting of this species by previous owners because it is currently among the highest values for sawlogs in Indiana (Settle and Gonso 2019). The species with the second and fourth highest importance values (Quercus bicolor Willd. and Prunus serotina Ehrh.) are known associates of J. nigra (Williams 1990). Standing dead trees had the third highest importance value in the overstory survey. These are essential in providing wildlife roosting sites and may provide insight into the relatively low percentage of canopy cover. Acer saccharum L., which shared the fourth highest importance value with P. serotina, is commonly the dominant species in second-growth forests in northeastern Indiana (e.g., Arvola et al. 2014, Bisht et al. 2017, Harman et al. 2019), which is why its lower rank at LWRNP was surprising.
Overall, LWRNP provides habitat to a relatively large pool of plant species (251 species across the three strata). Due to fragmentation, isolation, and diminished size of forests in the region, this property is of importance to preserving species and habitat. LWRNP provides an example of the plant diversity can exist in a small, protected forest. While the forest has been manipulated and its community structure dominated by human influence, there is still conservation value in continued protection of this site.
Acknowledgments
We would like to thank Liz King and Zach Heck for assistance in field data collection and Scott Namestnik (Indiana Department of Natural Resources, Division of Nature Preserves, Natural Heritage Program) for species identification verification, especially for Spiranthes species. We appreciate the comments and corrections provided by the editor-in-chief and the anonymous reviewers.
Appendix 1. Full list of species encountered at Little Wabash River Nature Preserve in the ecological surveys (column E) and the floristic surveys (column F). Family and scientific names follow
| Family | Scientific name | E | F | Voucher Number |
|---|---|---|---|---|
| Adoxaceae | Sambucus canadensis L. | X | X | LWRNP0109 |
| Alismataceae | Alisma subcordatum Raf. | X | LWRNP0077 | |
| Altingiaceae | Liquidambar styraciflua L. | X | ||
| Amaryllidaceae | Allium tricoccum Sol. var. burdickii Hanes | X | LWRNP0137 | |
| Anacardiaceae | Rhus glabra L. | X | ||
| Anacardiaceae | Rhus typhina L. | X | LWRNP0110 | |
| Anacardiaceae | Toxicodendron radicans (L.) Kuntze | X | X | |
| Annonaceae | Asimina triloba (L.) Dunal | X | ||
| Apiaceae | Cryptotaenia canadensis (L.) DC. | X | X | LWRNP0051 |
| Apiaceae | †Daucus carota L. | X | X | LWRNP0139 |
| Apiaceae | Erigenia bulbosa (Michx.) Nutt. | X | X | LWRNP0003 |
| Apiaceae | Osmorhiza claytonii (Michx.) C.B. Clarke | X | ||
| Apiaceae | Osmorhiza longistylis (Torr.) DC. | X | LWRNP0224 | |
| Apiaceae | †Pastinaca sativa L. | X | ||
| Apiaceae | Sanicula canadensis L. | X | X | LWRNP0093 |
| Apocynaceae | Apocynum cannabinum L. | X | X | LWRNP0108 |
| Apocynaceae | Asclepias incarnata L. | X | ||
| Apocynaceae | Asclepias quadrifolia Jacq. | X | ||
| Apocynaceae | Asclepias syriaca L. | X | X | LWRNP0078 |
| Araceae | Arisaema triphyllum (L.) Schott | X | LWRNP0031 | |
| Asparagaceae | †Asparagus officinalis L. | X | LWRNP0174 | |
| Asparagaceae | †Convallaria majalis L. | X | X | LWRNP0043 |
| Asparagaceae | Maianthemum racemosum (L.) Link | X | X | LWRNP0053 |
| Asparagaceae | Polygonatum biflorum (Walter) Elliott | X | X | LWRNP0047 |
| Aspleniaceae | Asplenium platyneuron (L.) Britton, Sterns & Poggenb. | X | X | |
| Asteraceae | Achillea millefolium L. | X | LWRNP0144 | |
| Asteraceae | Ageratina altissima (L.) R.M. King & H. Rob | X | X | LWRNP0189 |
| Asteraceae | Ambrosia artemisiifolia L. | X | X | LWRNP0193 |
| Asteraceae | Ambrosia trifida L. | X | ||
| Asteraceae | Antennaria parlinii Fernald subsp. fallax (Greene) R.J. Bayer & Stebbins | X | LWRNP0012 | |
| Asteraceae | †Arctium minus (Hill) Bernh. | X | ||
| Asteraceae | Bidens frondosa L. | X | LWRNP0232 | |
| Asteraceae | †Cichorium intybus L. | X | ||
| Asteraceae | †Cirsium arvense (L.) Scop. | X | X | LWRNP0083 |
| Asteraceae | †Cirsium vulgare (Savi) Ten. | X | ||
| Asteraceae | Eclipta prostrata (L.) L. | X | LWRNP0239 | |
| Asteraceae | Erigeron annuus (L.) Pers. | X | X | LWRNP0237 |
| Asteraceae | Erigeron philadelphicus L. | X | LWRNP0241 | |
| Asteraceae | Eupatorium perfoliatum L. | X | LWRNP0238 | |
| Asteraceae | Euthamia graminifolia (L.) Nutt. | X | X | LWRNP0236 |
| Asteraceae | Eutrochium maculatum (L.) E.E. Lamont | X | ||
| Asteraceae | Helianthus decapetalus L. | X | LWRNP0240 | |
| Asteraceae | †Hemerocallis fulva (L.) L. | X | LWRNP0087 | |
| Asteraceae | †Hieracium piloselloides Vill. | X | LWRNP0023 | |
| Asteraceae | Lactuca biennis (Moench) Fernald | X | LWRNP0185 | |
| Asteraceae | †Leucanthemum vulgare Lam. | X | X | LWRNP0170 |
| Asteraceae | Packera glabella (Poir) C. Jeffrey | X | ||
| Asteraceae | Solidago altissima L. | X | X | LWRNP0235 |
| Asteraceae | Solidago canadensis L. var. hargeri Fernald | X | X | LWRNP0234 |
| Asteraceae | Solidago sp. | X | ||
| Asteraceae | Symphyotrichum cordifolium (L.) G.L. Nesom | X | LWRNP0243 | |
| Asteraceae | Symphyotrichum lanceolatum (Willd.) G.L. Nesom | X | X | LWRNP0165 |
| Asteraceae | Symphyotrichum lateriflorum (L.) Á. Löve & D. Löve | X | LWRNP0242 | |
| Asteraceae | Symphyotrichum novae-angliae (L.) G.L. Nesom | X | LWRNP0156 | |
| Asteraceae | Symphyotrichum pilosum (Willd.) G.L. Nesom | X | LWRNP0168 | |
| Asteraceae | Symphyotrichum shortii (Lindl.) G.L. Nesom | X | X | LWRNP0157 |
| Asteraceae | †Taraxacum officinale F.H. Wigg. | X | X | LWRNP0227 |
| Asteraceae | Verbesina alternifolia (L.) Britton ex Kearney | X | ||
| Asteraceae | Vernonia gigantea (Walter) Trel. | X | X | LWRNP0230 |
| Balsaminaceae | Impatiens capensis Meerb. | X | X | LWRNP0138 |
| Berberidaceae | Podophyllum peltatum L. | X | LWRNP0049 | |
| Betulaceae | Betula papyrifera Marshall | X | ||
| Betulaceae | Carpinus caroliniana Walter | X | LWRNP0197 | |
| Betulaceae | Ostrya virginiana (Mill.) K. Koch | X | LWRNP0121 | |
| Boraginaceae | Hackelia virginiana (L.) I.M. Johnst. | X | LWRNP0141 | |
| Brassicaceae | †Alliaria petiolata (M. Bieb.) Cavara & Grande | X | X | LWRNP0036 |
| Brassicaceae | †Barbarea vulgaris W.T. Aiton | X | LWRNP0213 | |
| Brassicaceae | †Brassica nigra (L.) W.D.J. Koch | X | ||
| Brassicaceae | Cardamine concatenata (Michx.) Sw. | X | X | LWRNP0006 |
| Brassicaceae | Cardamine douglassii Britton | X | X | LWRNP0015 |
| Brassicaceae | †Lepidium campestre (L.) W.T. Aiton | X | X | LWRNP0035 |
| Campanulaceae | Campanulastrum americanum (L.) Small | X | LWRNP0132 | |
| Campanulaceae | Lobelia inflata L. | X | LWRNP0175 | |
| Campanulaceae | Lobelia siphilitica L. | X | LWRNP0195 | |
| Cannabaceae | Celtis occidentalis L. | X | X | LWRNP0113 |
| Caprifoliaceae | †Lonicera maackii (Rupr.) Herder | X | X | LWRNP0074 |
| Caprifoliaceae | †Lonicera sp. | X | LWRNP0112 | |
| Caryophyllaceae | †Cerastium fontanum Baumg. | X | X | LWRNP0177 |
| Caryophyllaceae | †Dianthus armeria L. | X | LWRNP0204 | |
| Caryophyllaceae | †Stellaria media (L.) Vill. | X | X | LWRNP0225 |
| Celastraceae | Euonymus atropurpureus Jacq. | X | ||
| Convulvulaceae | †Calystegia silvatica (Kit.) Griseb. | X | LWRNP0167 | |
| Cornaceae | Cornus drummondii C.A. Mey | X | X | LWRNP0104 |
| Cornaceae | Cornus racemosa Lam. | X | X | LWRNP0084 |
| Cucurbitaceae | Echinocystis lobata (Michx.) Torr. & A. Gray | X | LWRNP0172 | |
| Cupressaceae | Juniperus virginiana L. | X | ||
| Cupressaceae | Taxodium distichum (L.) Rich. var. distichum | X | ||
| Cyperaceae | Carex blanda Dewey | X | LWRNP0194 | |
| Cyperaceae | Carex granularis Muhl. ex Willd. | X | X | LWRNP0181 |
| Cyperaceae | Carex hirtifolia Mack. | X | LWRNP0190 | |
| Cyperaceae | Carex jamesii Schwein. | X | X | LWRNP0044 |
| Cyperaceae | Carex laevivaginata (Kük.) Mack. | X | LWRNP0082 | |
| Cyperaceae | Carex normalis Mack. | X | X | LWRNP0183 |
| Cyperaceae | Carex oligocarpa Schkuhr ex Willd. | X | LWRNP0208 | |
| Cyperaceae | Carex rosea Schkuhr ex Willd. | X | LWRNP0199 | |
| Cyperaceae | Carex shortiana Dewey | X | LWRNP0179 | |
| Cyperaceae | Carex sparganioides Muhl. ex Willd. | X | LWRNP0205 | |
| Cyperaceae | Carex stipata Muhl. ex Willd. | X | X | LWRNP0196 |
| Cyperaceae | Carex tribiloides Wahlenb. | X | ||
| Cyperaceae | Carex vulpinoidea Michx. | X | X | LWRNP0201 |
| Cyperaceae | Scirpus atrovirens Willd. | X | X | LWRNP0130 |
| Dioscoreaceae | Dioscorea villosa L. | X | ||
| Dryopteridaceae | Dryopteris carthusiana (Vill.) H.P. Fuchs | X | LWRNP0056 | |
| Dryopteridaceae | Polystichum acrostichoides (Michx.) Schott | X | LWRNP0055 | |
| Ebenaceae | Diospyros virginiana L. | X | ||
| Elaeagnaceae | †Elaeagnus umbellata Thunb. | X | X | LWRNP0026 |
| Equisetaceae | Equisetum arvense L. | X | LWRNP0009 | |
| Ericaceae | Monotropa uniflora L. | X | LWRNP0192 | |
| Fabaceae | Amphicarpaea bracteata (L.) Fernald | X | LWRNP0166 | |
| Fabaceae | Cercis canadensis L. | X | X | LWRNP0118 |
| Fabaceae | Desmodium paniculatum (L.) DC. | X | LWRNP0202 | |
| Fabaceae | Gleditsia triacanthos L. | X | LWRNP0101 | |
| Fabaceae | †Medicago sativa L. | X | ||
| Fabaceae | †Securigera varia (L.) Lassen | X | LWRNP0114 | |
| Fabaceae | †Trifolium pratense L. | X | X | LWRNP0052 |
| Fabaceae | †Trifolium repens L. | X | X | LWRNP0146 |
| Fagaceae | Quercus alba L. | X | X | |
| Fagaceae | Quercus bicolor Willd. | X | X | LWRNP0075 |
| Fagaceae | Quercus coccinea Münchh. | X | ||
| Fagaceae | Quercus muehlenbergii Engelm. | X | ||
| Fagaceae | Quercus rubra L. | X | X | LWRNP0091 |
| Papaveraceae | Dicentra canadensis (Goldie) Walp. | X | LWRNP0008 | |
| Papaveraceae | Dicentra cucullaria (L.) Bernh. | X | LWRNP0001 | |
| Geraniaceae | Geranium maculatum L. | X | X | LWRNP0038 |
| Grossulariaceae | Ribes cynosbati L. | X | X | LWRNP0014 |
| Hydrophyllaceae | Hydrophyllum appendiculatum Michx. | X | X | LWRNP0048 |
| Hydrophyllaceae | Hydrophyllum macrophyllum Nutt. | X | X | LWRNP0057 |
| Hydrophyllaceae | Hydrophyllum virginianum L. | X | LWRNP0218 | |
| Hydrophyllaceae | Phacelia bipinnatifida Michx. | X | ||
| Hypericaceae | †Hypericum perforatum L. | X | LWRNP0134 | |
| Hypericaceae | Hypericum punctatum Lam. | X | LWRNP0217 | |
| Iridaceae | Sisyrinchium angustifolium Mill. | X | LWRNP0028 | |
| Juglandaceae | Carya cordiformis (Wangenh.) K. Koch | X | X | |
| Juglandaceae | Carya ovata (Mill.) K. Koch | X | ||
| Juglandaceae | Juglans nigra L. | X | ||
| Juncaceae | Juncus tenuis Willd. | X | X | LWRNP0067 |
| Lamiaceae | Agastache nepetoides L. | X | LWRNP0184 | |
| Lamiaceae | Blephilia hirsuta (Pursh) Benth. | X | ||
| Lamiaceae | Collinsonia canadensis L. | X | LWRNP0182 | |
| Lamiaceae | †Glechoma hederacea L. | X | X | LWRNP0050 |
| Lamiaceae | Lycopus americanus Muhl. ex W.P.C. Barton | X | LWRNP0200 | |
| Lamiaceae | Monarda fistulosa L. | X | LWRNP0097 | |
| Lamiaceae | Monarda serotina nom. illeg. | X | LWRNP0250 | |
| Lamiaceae | †Origanum vulgare L. | X | LWRNP0088 | |
| Lamiaceae | Prunella vulgaris L. | X | X | LWRNP0090 |
| Lamiaceae | Stachys tenuifolia Willd. | X | LWRNP0224 | |
| Lamiaceae | Teucrium canadense L. | X | LWRNP0092 | |
| Lauraceae | Lindera benzoin (L.) Blume | X | LWRNP0158 | |
| Liliaceae | Erythronium albidum Nutt. | X | LWRNP0007 | |
| Liliaceae | Erythronium americanum Ker Gawl. | X | X | LWRNP0002 |
| Limnanthaceae | Floerkea proserpinacoides Willd. | X | X | LWRNP0032 |
| Magnoliaceae | Liriodendron tulipifera L. | X | LWRNP0073 | |
| Malvaceae | Tilia americana L. | X | LWRNP0105 | |
| Melanthiaceae | Trillium sessile L. | X | LWRNP0010 | |
| Menispermaceae | Menispermum canadense L. | X | X | LWRNP0058 |
| Montiaceae | Claytonia virginica L. | X | X | LWRNP0005 |
| Moraceae | †Morus nigra L. | X | ||
| Moraceae | Morus rubra L. | X | LWRNP0020 | |
| Myrsinaceae | Lysimachia quadrifolia L. | X | LWRNP0122 | |
| Oleaceae | Fraxinus pennsylvanica Marshall | X | ||
| Oleaceae | Fraxinus quadrangulata Michx. | X | X | LWRNP0159 |
| Onagraceae | Circaea canadensis (L.) Hill | X | X | LWRNP0145 |
| Onagraceae | Circaea lutetiana L. | X | ||
| Onagraceae | Epilobium coloratum Biehler | X | LWRNP0169 | |
| Onagraceae | Ludwigia palustris (L.) Elliott | X | LWRNP0215 | |
| Onocleaceae | Onoclea sensibilis L. | X | LWRNP0054 | |
| Ophioglossaceae | Botrychium virginianum (L.) Sw. | X | LWRNP0080 | |
| Ophioglossaceae | Ophioglossum vulgatum L. | X | LWRNP0076 | |
| Orchidaceae | Liparis liliifolia (L.) Rich. ex Ker Gawl. | X | ||
| Orchidaceae | Spiranthes cernua (L.) Rich. | X | LWRNP0251 | |
| Orchidaceae | Spiranthes lacera (Raf.) Raf. var. gracilis (Bigelow) Luer | X | LWRNP0162 | |
| Orchidaceae | Spiranthes magnicamporum Sheviak | * | ||
| Orchidaceae | Spiranthes ovalis Lind. | * | ||
| Oxalidaceae | Oxalis dillenii Jacq. | X | X | LWRNP0143 |
| Papaveraceae | Sanguinaria canadensis L. | X | ||
| Penthoraceae | Penthorum sedoides L. | X | LWRNP0149 | |
| Phrymaceae | Mimulus ringens L. | X | LWRNP0180 | |
| Phrymaceae | Phryma leptostachya L. | X | X | LWRNP0136 |
| Phytolaccaceae | Phytolacca americana L. | X | LWRNP0178 | |
| Pinaceae | Picea glauca (Moench) Voss | X | ||
| Pinaceae | Pinus strobus L. | X | X | |
| Plantaginaceae | †Plantago lanceolata L. | X | X | LWRNP0024 |
| Plantaginaceae | Plantago rugelii Decne. | X | ||
| Plantaginaceae | †Veronica serpyllifolia L. subsp. serpyllifolia | X | LWRNP0220 | |
| Platanaceae | Platanus occidentalis L. | X | ||
| Poaceae | †Agrostis gigantea Roth | X | X | LWRNP0207 |
| Poaceae | †Bromus inermis Leyss. | X | LWRNP0216 | |
| Poaceae | Bromus pubescens Muhl. ex Willd. | X | X | LWRNP0081 |
| Poaceae | †Dactylis glomerata L. | X | X | LWRNP0210 |
| Poaceae | Dichanthelium boscii (Poir.) Gould & C.A. Clark | X | X | LWRNP0085 |
| Poaceae | Dichanthelium linearifolium (Scribn. ex Nash) Gould | X | ||
| Poaceae | †Echinochloa crus-galli (L.) P. Beauv. | X | ||
| Poaceae | Elymus canadensis L. | X | LWRNP0071 | |
| Poaceae | Elymus hystrix L. | X | X | LWRNP0129 |
| Poaceae | Elymus virginicus L. var. virginicus | X | LWRNP0209 | |
| Poaceae | Glyceria striata (Lam.) Hitchc. | X | X | LWRNP0249 |
| Poaceae | Leersia virginica Willd. | X | LWRNP0219 | |
| Poaceae | †Phleum pratense L. | X | X | LWRNP0068 |
| Poaceae | tPoa pratensis L. subsp. pratensis | X | X | LWRNP0221 |
| Poaceae | Poa sylvestris A. Gray | X | X | LWRNP0223 |
| Polemoniaceae | Phlox divaricata L. | X | LWRNP0039 | |
| Polemoniaceae | Polemonium reptans L. | X | LWRNP0037 | |
| Polygonaceae | Fallopia scandens (L.) Holub | X | LWRNP0173 | |
| Polygonaceae | Persicaria punctata (Elliott) Small | X | LWRNP0247 | |
| Polygonaceae | Persicaria virginiana (L.) Gaertn | X | X | LWRNP0176 |
| Primulaceae | Lysimachia ciliata L. | X | LWRNP0229 | |
| Ranunculaceae | Actaea pachypoda Elliott | X | X | LWRNP0046 |
| Ranunculaceae | Anemone canadensis L. | X | ||
| Ranunculaceae | Ranunculus abortivus L. | X | X | LWRNP0016 |
| Ranunculaceae | †Ranunculus ficaria L. | X | LWRNP0030 | |
| Ranunculaceae | Ranunculus hispidus Michx. | X | LWRNP0045 | |
| Ranunculaceae | Ranunculus recurvatus Poir. | X | X | LWRNP0017 |
| Rosaceae | Agrimonia pubescens Wallr. | X | LWRNP0228 | |
| Rosaceae | Crataegus sp. | X | LWRNP0233 | |
| Rosaceae | †Duchesnea indica (Andrews) Focke var. indica | X | ||
| Rosaceae | Fragaria vesca L. | X | ||
| Rosaceae | Fragaria vesca L. subps. vesca | X | ||
| Rosaceae | Fragaria virginiana Duchesne subsp. grayana (E. Vilm. ex J. Gray) Staudt | X | LWRNP0059 | |
| Rosaceae | Geum canadense Jacq. | X | X | LWRNP0065 |
| Rosaceae | Geum sp. | X | ||
| Rosaceae | Geum vernum (Raf.) Torr. & A. Gray | X | X | LWRNP0040 |
| Rosaceae | †Potentilla recta L. | X | LWRNP0135 | |
| Rosaceae | Prunus serotina Ehrh. | X | X | LWRNP0107 |
| Rosaceae | †Rosa multiflora Thunb. | X | X | LWRNP0212 |
| Rosaceae | Rosa setigera Michx. var. tomentosa Torr. & A. Gray | X | LWRNP0246 | |
| Rosaceae | Rosa sp. | X | LWRNP0248 | |
| Rosaceae | Rubus occidentalis L. | X | X | LWRNP0211 |
| Rubiaceae | Galium aparine L. | X | X | LWRNP0061 |
| Rubiaceae | Galium asprellum Michx. | X | ||
| Rubiaceae | Galium circaezans Michx. | X | X | LWRNP0063 |
| Rubiaceae | Galium concinnum Torr. & A. Gray | X | X | LWRNP0086 |
| Rubiaceae | Galium triflorum Michx. | X | X | LWRNP0119 |
| Salicaceae | Populus deltoides W. Bartram ex Marshall | X | ||
| Salicaceae | Salix nigra Marshall | X | LWRNP0111 | |
| Sapindaceae | Acer negundo L. | X | LWRNP0198 | |
| Sapindaceae | Acer saccharinum L. | X | ||
| Sapindaceae | Acer saccharum Marshall | X | X | LWRNP0019 |
| Sapindaceae | Aesculus glabra Willd. | X | X | LWRNP0072 |
| Scrophulariaceae | Scrophularia marilandica L. | X | ||
| Smilacaceae | Smilax ecirrhata S. Watson | X | LWRNP0100 | |
| Smilacaceae | Smilax sp. | X | ||
| Smilaceae | Smilax tamnoides L. | X | X | LWRNP0226 |
| Solanaceae | Physalis longifolia Nutt. | X | LWRNP0131 | |
| Solanaceae | Solanum carolinense L. | X | X | LWRNP0245 |
| Ulmaceae | Ulmus americana L. | X | X | LWRNP0120 |
| Ulmaceae | Ulmus rubra Muhl. | X | LWRNP0152 | |
| Urticaceae | Boehmeria cylindrica (L.) Sw. | X | X | LWRNP0186 |
| Urticaceae | Laportea canadensis (L.) Benth. | X | X | |
| Urticaceae | Pilea pumila (L.) A. Gray | X | X | LWRNP0140 |
| Urticaceae | Urtica dioica L. | X | ||
| Verbenaceae | Verbena urticafolia L. | X | ||
| Violaceae | Viola pubescens Aiton | X | LWRNP0011 | |
| Violaceae | Viola sororia Willd. | X | X | LWRNP0095 |
| Violaceae | Viola striata Aiton | X | X | LWRNP0093 |
| Vitaceae | Parthenocissus quinquefolia (L.) Planch. | X | X | LWRNP0034 |
| Vitaceae | Vitis vulpina L. | X | X | LWRNP0222 |
Literature Cited
Arvola, K. D., P. F. Booth, C. C. Ellinwood, A. J. Fry, J. L. Furge, K. A. Haehnle, L. E. Hall, et al. (2014). Comparative analysis of urban and rural forest fragment structure and diversity in Northeastern Indiana. The Michigan Botanist 53: 39–59.
Bisht, A., K. L. Fracica, A. M. Gutierrez, J. R. Hinson, A. A. Hopkins, J. M. Josimovich, M. N. Matthews, et al. (2017). Survey of Haskamp Woods, Allen County, Indiana, and floristic comparison with neighboring forest properties. Phytoneuron 2017- 69: 1–13.
Diamond, J. M., and J. T. Heinen. (2016). Conserving rare plants in locally-protected urban forest fragments: A case study from Miami-Dade County, Florida. Urban Forestry & Urban Greening 20: 1–11.
Eyre, F. H. (1980). Forest cover types of the United States and Canada. Society of American Foresters, Washington, D.C.
Fauth, P. T. (2000). Reproductive success of wood thrushes in forest fragments in northern Indiana. The Auk 117: 194–204.
Fleming, A. H., J. O. Farlow, A. Argast, G. M. Grammer, and D. Prezbindowski. (2018). The Maumee Megaflood and the geomorphology, environmental geology, and Silurian-Holocene history of the upper Wabash Valley and vicinity, north-central Indiana. Pp. 259–337 in Ancient oceans, orogenic uplifts, and glacial ice: Geologic crossroads in America’s heartland. L. J. Florea, editor. Geological Society of America, Boulder, Colorado.
Freemark, K. E., and H. G. Merriam. (1986). Importance of area and habitat heterogeneity to bird assemblages in temperate forest fragments. Biological Conservation 36: 115–141.
Harman, R. R., A. R. De Leon, H. M. Lancaster, and J. M. Marshall. (2019). Relative influence of spatial and structural characteristics of forest fragments on woody plant communities. The Great Lakes Botanist 58: 32–56.
Harper, K. A., S. E. MacDonald, P. J. Burton, J. Chen, K. D. Brosofske, S. C. Saunders, E. S. Euskirchen, et al. (2005). Edge influence on forest structure and composition in fragmented landscapes. Conservation Biology 19: 768–782.
Hayek, L. C., and M. A. Buzas. (1997). Surveying natural populations. Columbia University Press, New York, N.Y.
ITIS. (2023). Integrated Taxonomic Information System. Available at https://www.itis.gov (Accessed July 31, 2023).https://www.itis.gov
Lemon, P. E. (1956). A spherical densiometer for estimating forest overstory density. Forest Science 2: 314–320.
Mattrick, C. (2004). Liparis liliifolia (L.) L. C. Rich. ex Lindley, Lily-leaved twayblade: Conservation and research plan for New England. New England Plant Conservation Program, New England Wild Flower Society. Available at https://www.nativeplanttrust.org/documents/75/Liparis_liliifolia.pdf (Accessed October 10, 2022).https://www.nativeplanttrust.org/documents/75/Liparis_liliifolia.pdf
Morris, M. W. (1989). Spiranthes (Orchidaceae) in Mississippi. Selbyana 11: 39–48.
Myers, A. L., and J. M. Marshall. (2021). Influence of forest fragment composition and structure on ground-dwelling arthropod communities. The American Midland Naturalist 186: 76–94.
Nupp, T. E., and R. K. Swihart. (2000). Landscape-level correlates of small-mammal assemblages in forest fragments of farmland. Journal of Mammalogy 81: 512–526.
Oksanen, J., G. Simpson, F. Blanchet, R. Kindt, P. Legendre, P. Minchin, R. O’Hara, et al. (2022). vegan: Community ecology package. R package version 2.6–2. Available at https://CRAN.R-project.org/package=vegan (Accessed July 31, 2023).https://CRAN.R-project.org/package=vegan
Proesmans, W., D. Bonte, G. Smagghe, I. Meeus, and K. Verheyen. (2019). Importance of forest fragments as pollinator habitat varies with season and guild. Basic and Applied Ecology 34: 95–107.
R Core Team. (2022). R: A language and environment for statistical computing. R Foundation of Statistical Computing, Vienna, Austria.
Rooney, T. P., and D. A. Rogers. (2002). The modified floristic quality index. Natural Areas Journal 22: 340–344.
Rosenblatt, D. L., E. J. Heske, S. L. Nelson, D. M. Barber, M. A. Miller, and B. MacAllister. (1999). Forest fragments in east-central Illinois: Islands or habitat patches for mammals? The American Midland Naturalist 141: 115–123.
Rothrock, P. E. (1997). The vascular flora of Fogwell Forest Nature Preserve, Allen County, Indiana. Proceedings of the Indiana Academy of Science 106: 267–290.
Rothrock, P. E. (2004). Floristic quality assessment in Indiana: The concept, use, and development of coefficients of conservatism. Final report for ARN A305–4-53, EPA Wetland Program Development Grant CD975586–01. Indiana Department of Environmental Management, Office of Water Quality, Indianapolis, Indiana, USA. Available online at https://www.lrl.usace.army.mil/Portals/64/docs/regulatory/FloristicAssessment_IND.pdf (Accessed July 31, 2023).https://www.lrl.usace.army.mil/Portals/64/docs/regulatory/FloristicAssessment_IND.pdf
Rothrock, P. E., and M. A. Homoya. (2005). An evaluation of Indiana’s floristic quality assessment. Proceedings of the Indiana Academy of Science 114: 9–18
Settle, J., and C. Gonso. (2019). 2019 Indiana forest products price report and trend analysis. The Woodland Steward 28: 1–5.
Spyreas, G. (2019). Floristic quality assessment: A critique, a defense, and a primer. Ecosphere 10: e02825.
Swink, F., and G. Wilhelm. (1994). Plants of the Chicago region. 4th edition. Indiana Academy of Science, Indianapolis, Indiana.
Warners, D. P., G. E. Crow, J. D. Walt, C. R. Koehn, Z. E. Hartwig, and D. Clum. (2021). Botanical assessment of high-quality woodland parcels in the undeveloped Lowell Regional Greenspace, Kent County, Michigan. The Great Lakes Botanist 60: 110–148.
Wilhelm, G. S., T. P. Simon, and P. M. Stewart. (2003). Conservatism of confined disposal facilities based on the biological stability and integrity of plant communities: A case study in the Laurentian Great Lakes basin. Pp. 251–269 in Biological response signatures: Indicator patterns using aquatic communities. T. P. Simon, editor. CRC Press, Boca Raton, Florida.
Wilhite, L. P., and J. R. Toliver. (1990). Taxodium distichum (L.) Rich. Baldcypress. Pp. 563–572 in Silvics of North America, Volume 1: Conifers. R. M. Burns and B. H. Honkala, technical coordinators. Agriculture Handbook 654. United States Department of Agriculture, Forest Service, Washington, D.C. Available at https://www.srs.fs.usda.gov/pubs/misc/ag_654/volume_1/taxodium/distichum.htm (Accessed October 10, 2022).https://www.srs.fs.usda.gov/pubs/misc/ag_654/volume_1/taxodium/distichum.htm
Williams, R. D. (1990). Juglans nigra L. Black walnut. Pp. 391–399 in Silvics of North America, Volume 2: Hardwoods. R. M. Burns and B. H. Honkala, technical coordinators. United States Department of Agriculture, Forest Service. Agriculture Handbook 654. United States Department of Agriculture, Forest Service, Washington, D.C. Available at https://www.srs.fs.usda.gov/pubs/ misc/ag_654/volume_2/juglans/nigra.htm (Accessed October 10, 2022).https://www.srs.fs.usda.gov/pubs/ misc/ag_654/volume_2/juglans/nigra.htm