INTRODUCTION
Wild lands around the globe are facing numerous challenges from anthropogenic disturbance, invasive species, and pollution as well as from climate-related natural disasters like wildfire, drought, or floods. Ecological restoration, the process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed (Society for Ecological Restoration International Science & Policy Working Group 2004), is becoming essential to maintain robust ecological communities, the biodiversity they support, and the ecosystem services they provide. As we begin the United Nations Decade on Ecosystem Restoration 2021–2030 (UNEP 2024), ecological restoration has become a high priority, as highlighted in numerous national—e.g., the Biden Administration’s “America the Beautiful Initiative”— (USDOI 2024) and international policy documents— e.g., the Global Strategy for Plant Conservation (CBD 2024), the Sustainable Development Goals (UN 2015), and the Bonn Challenge (IUCN 2020). Since the majority of restoration projects involve the introduction of seeds or seedlings, one of the ongoing challenges is determining a provenancing strategy for sourcing seeds or plant material to ensure it is well-adapted to the site (Broadhurst et al. 2008; Prober et al. 2015; Breed et al. 2018).
Local adaptation is well-documented in plants (Leimu and Fischer 2008; Hereford 2009), and the assumption that “local is best” has guided seed sourcing decisions for decades (Mortlock 2000; McKay et al. 2005). However, rapidly changing climate has led restoration practitioners to question these assumptions as local seed sources may be better adapted to historical or contemporary climates than to future ones (Broadhurst et al. 2008; Havens et al. 2015; Prober et al. 2015; Breed et al. 2018). Seed transfer zones (the areas from which germplasm can be moved and still be well-adapted to the climate) are influenced by many species-specific and site-specific factors, including patterns of gene flow, endemism, and the heterogeneity and stability of the landscape (Johnson et al. 2004; Havens et al. 2015). If restored populations are to survive projected future climates, seed transfer zones will need to become more dynamic (Kramer and Havens 2009). As the recognition of the need to prepare for future climates increases, several alternative strategies to local provenancing have been suggested. These strategies generally either increase the genetic diversity of the source material (and therefore presumably the adaptive potential of the plants) or attempt to match seed source with anticipated future climate at a restoration site (Breed et al. 2018). Despite many years of robust discussion in the literature of the pros and cons of various provenancing strategies, which we understand can be challenging to implement in practice, empirical studies are still limited.
Central to the provenancing debate are not only the direct effects on the restoration related to the climatic fit of the plant material to the site, but also indirect effects on ecological function, including plant–animal interactions (Hobbs and Cramer 2008; Genes and Dirzo 2022). Plant phenology, as dictated by genetic and environmental variables, defines the temporal overlap and hence the potential for direct interaction across trophic levels (Durant et al. 2005; Peralta et al. 2020). Rapid anthropogenic climate change, including increases in mean temperature as well as in climate extremes and variability, has been linked to phenological shifts, such as earlier leaf out and flowering (Anderson et al. 2012; Piao et al. 2019). As species can respond independently to climatic shifts, there is a potential for plant–pollinator mismatch, or for divergent timing of flowering and pollinator life cycles, which can reduce fitness in plant and/or pollinator (Memmott et al. 2007; Forrest 2014; Howard 2018). Recent, expanded investigations of the impact of seed source have begun to document effects on species interactions and associated communities (Bucharova et al. 2016; Gehring et al. 2017; Gosney et al. 2017), including the relationship between plant phenology and pollinator networks (Bucharova et al. 2021). As restoration aims to reestablish interactions as well as organisms, an expanded understanding of the impact of seed source on plant performance and ecology is key to a comprehensive and resilient ecological restoration (Breed et al. 2018).
Since the relationship between seed source and pollinators are not one-way, but a mutualism, we would expect to see impacts on plant fitness and hence the successful establishment of a self-sustaining, restored plant community. To investigate the impact of seed source on plant phenology, pollination, and reproduction, we conducted a three-year common garden trial for Asclepias syriaca L. (common milkweed, Apocynaceae) within the core of its native range in the midwestern United States. This species is of particular relevance given its central role as a larval host in the life cycle of the imperiled Danaus plexippus L. (monarch butterfly), a charismatic pollinator species that has garnered international attention over the last decade (Brower et al. 2012; Trudeau et al. 2016; Thogmartin et al. 2017a). The study design allowed for a simultaneous test of interactions between seed source and pollinators as well as between seed source and climate, within which we tested four related hypotheses:
H1 Flowering Phenology – Flowering phenology varies among seed sources and follows source latitude, with northern sources flowering earlier than southern sources.
H2 Floral Visitation – Floral visitation varies among seed sources and mirrors flowering phenology, with increased floral visitation during peak flowering.
H3 Plant Size – Plant height at maturity varies among seed sources and follows source latitude, with northern sources reaching shorter maximum heights than southern sources.
H4 Reproduction – Fruit set varies among seed sources and follows source latitude, with northern sources generating less reproductive output than southern sources.
MATERIALS AND METHODS
Study Species
Milkweeds (Asclepias spp.) are characterized by a unique floral morphology and milky, alkaloidrich latex that has driven coevolution with many insect herbivores, most famously the monarch butterfly, which depends on milkweed as a larva host plant to complete its lifecycle (Malcolm 1994). Over the last 20 years, dramatic declines have been observed in the overwintering size of the eastern migratory population of monarch butterflies in North America (Semmens et al. 2016). Monarch declines have been linked to a range of interrelated factors, including habitat loss, pesticide use, and climate change (Flockhart et al. 2014; Thogmartin et al. 2017b). In response there has been a marked increase in monarch habitat restoration efforts, including the recommendation to plant 1.8 billion stems of milkweed along their migratory route in the midwestern United States. (Pleasants 2017; Midwest Association of Fish and Wildlife Agencies 2018). Importantly, milkweed flowers lack an exposed stigmatic surface, preventing self-fertilization and requiring insect-mediated transfer of pollen packages (pollinia) through narrow floral openings called stigmatic slits. Plant species with obligate animal pollination syndromes, such as milkweeds, are expected to be more sensitive to changes in pollinator abundance and phenology as a result of climate change. Due to the wider interest in planting milkweed to bolster the monarch population, as well as its reproductive dependence on insect pollination, the relationship between seed source, phenology, and reproduction in milkweed was investigated. Specifically, common milkweed was studied, a generalist that thrives in marginal habitats, such as roadsides and agricultural field margins, which contributes to its status as one of the most abundant milkweed species in the upper Midwest (Hartzler and Buhler 2000). Results of stable isotope studies of overwintering monarch butterflies in Mexico support the assumption that common milkweed is one of the most important larval food sources (Seiber et al. 1986).
Seed Collection and Common Garden
In fall 2013, seed was collected from three naturally occurring populations of common milkweed in Minnesota, Illinois, and Missouri (Table 1). Seed collections were carried out at the time of seed dispersal with at least thirty individuals sampled per population. To maximize the likelihood of collecting from genetically distinct individuals, seeds were collected from stems spaced at least five meters apart. Seeds were cleaned manually by removing them from the pods and removing the comas. Seeds were then combined by source population. The seeds were germinated in production greenhouses at the Chicago Botanic Garden in Glencoe, Illinois in the winter of 2013–14. In spring 2014, a common garden was established at the Chicago Botanic Garden consisting of 144 individuals per seed source planted into a grid (24–30” spacing) to form three adjacent blocks (4.28 × 9.14m) following stratified random placement (42°08¢36.4¢¢N 87°47¢09.4¢¢W). In this context the Illinois seed source is considered to be local, with the Minnesota and Missouri sources considered to be northern and southern, respectively. The blocks were located within a garden bed that was surrounded by turf grass and that was next to ornamental trial garden beds to the north and south, a plant production area to the east, and a restored native shoreline to the west. The common garden was mulched with leaf mold, was regularly weeded to maintain bare ground between study plants, and was watered through aerial sprinkler irrigation to maintain normal precipitation for the region. The regular maintenance did not have any noticeable negative effects on the use of the study plants by insect herbivores.
Location of populations from which seeds were collected.
State |
Location |
Latitude (N) |
Longitude (W) |
|---|---|---|---|
Minnesota |
Minnesota Landscape Arboretum, Bennett-Johnson Prairie |
44° 51¢ 36¢¢ |
–93° 37¢ 33.6¢¢ |
Illinois |
Nachusa Grasslands |
41° 52¢ 51.6¢¢ |
–89° 20¢ 31.2¢¢ |
Missouri |
Roadside, west of the intersection of I-44 and Missouri Hwy 141 |
38° 32¢ 16.8¢¢ |
–90° 30¢ 7.2¢¢ |
Plant Phenology, Size, and Fruit Set
After an initial establishment year in 2015, phenology data were collected over two growing seasons: 2016 (all surviving plants; 109–123 plants/seed source = 350 total) and 2017 (random subset of plants; 30 plants/seed source = 90 total). At least once weekly, phenophase was recorded for each surviving individual in 2016 and thirty randomly tagged individuals per seed source in 2017. Seven stages (phenophases) were used to define individual phenology: vegetative, flower buds, first flower, early flower, full flower, post flower, and early fruiting. These stages are described in Table 2 and, other than the vegetative phase, are illustrated in Figure 1. At the end of the growing season (mid-September), the maximum height of each plant (soil to tallest point) and, for reproductive individuals, the number of follicles (hereafter, fruit) were recorded. Fruits were scored via visual assessment and recorded as viable (full size, rigid, green, not dehisced) or aborted (immature size, dry and pliant, grey to brown, dehisced without presence of apparently viable seeds). Milkweeds are known for producing many aborted fruits (Gaertner 1979; Stephenson 1981).
Phenophase is defined for each umbel (inflorescence) and averaged across all umbels on the plant to determine plant phenophase. Reference photos are provided in Figure 1.
Phenophase |
Description |
|---|---|
Vegetative |
No buds, flowers, or fruits present |
Flower buds |
Buds with green to light pink coloration, no open flowers |
First flower |
Buds with dark pink coloration with few open flowers (<10%) |
Early flower |
More than 10% but less than 50% of flower buds have opened |
Full flower |
>50% of flower buds have opened |
Post flower |
All buds have opened, flowers have become shriveled, color has changed to yellow to brown, and hang down from their pedicels |
Early fruiting |
Small, green fruits have begun to develop |
Floral Visitation
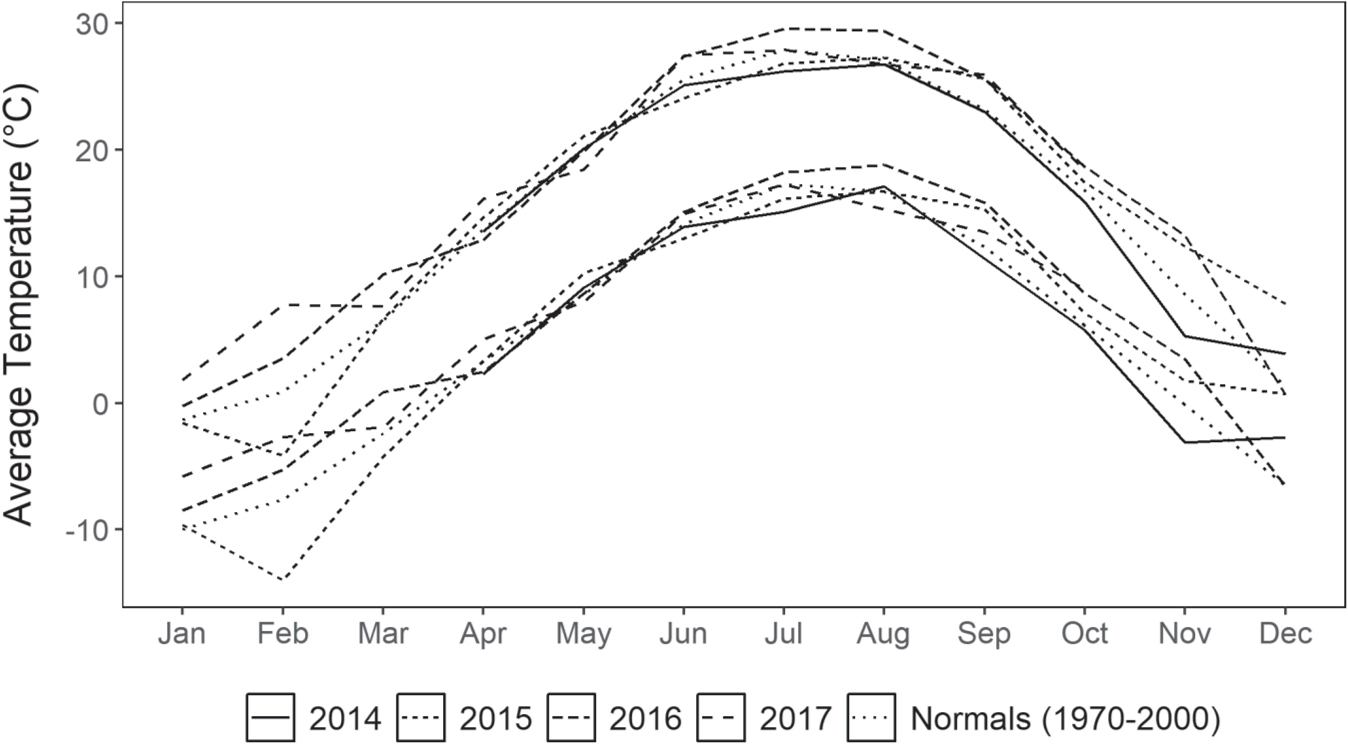
In year three (2017) pollinator observations were conducted two to three times per week during the flowering period, alternating between morning (10–12am) and afternoon (1–3pm) sessions on warm, sunny days (daily maximum 24–32°C; Chicago Botanic Garden unpublished data). During each session a 2×2m plot in each block was monitored for one 15-minute interval. Plots were randomly selected within each seed source using transects running north to south along the east and west sides of the common garden plot. To ensure visitation was not confounded by time of day, morning and afternoon sessions alternated between east and west transects. Within each observation plot, the length of visitation per plant, the number of plants visited, and the phenophase of both plant(s) and individual umbel(s) visited were recorded for each floral visitor. Observations were conducted in intervals of an average of 15 minutes each for a total of 563 minutes (37 sessions over 10 days) for all seed sources with 136 minutes (9 sessions over 5 days), 219 minutes (14 sessions over 10 days), and 208 minutes (14 sessions over 10 days) for the Minnesota, Illinois, and Missouri blocks, respectively. The Minnesota population had an abbreviated observation window, because its flowering period ended earlier. Average temperature during the course of the study (Apr 2014–Oct 2017, NOAA 2019) largely aligned with climate normals (1970–2000; Fick and Hijmans 2017) for the site (Figure 2). However, the winters of 2016 and 2017 were warmer than average, as were the falls of 2015 and 2016.
Statistical Analyses
A coarse estimate of the number of flowers per seed source was calculated as the sum of the number of viable and aborted fruits. Linear models were used to evaluate phenological variation among seed sources with respect to 1) day of peak flower, and 2) flowering duration. For both phenology models (1 and 2), the date was converted to day of year and due to disparate sample sizes only complete phenology data from 2016 was included. For model 1, the response variable was the average day of peak flower for an individual plant, and the predictor was seed source. For model 2, flowering duration was calculated for individual plants as the number of days from (and including) first flower to the day before post flower was first observed. Variation in floral visitation was tested using generalized linear models (family=quasipoisson) with maximal models including an additive relationship between seed source, observation period, and time of day (morning or afternoon). Variation in visit length (min:sec) by seed source and plant height were evaluated using linear models. Generalized linear models were used to investigate the variation among seed sources in fruit production (number of viable and aborted fruits, family=quasipoisson) and the proportion of viable fruit (family=quasibinomial). All maximal models for fruit set include seed source, year, and the interaction between the two. The best models were selected through backwards elimination, as described in Crawley (2015), and defined as the minimal adequate model to fit the data. Pair-wise significant differences among seed sources were evaluated using the ‘glht’ function for multiple comparisons in parametric models in the package ‘multcomp’ (type= ‘Tukey’) (Hothorn et al. 2017). All analyses were conducted in R: A language and environment for statistical computing (version 4.0.4, 2021-02-15) (R Core Team 2021).
RESULTS
Flowering Phenology and Duration
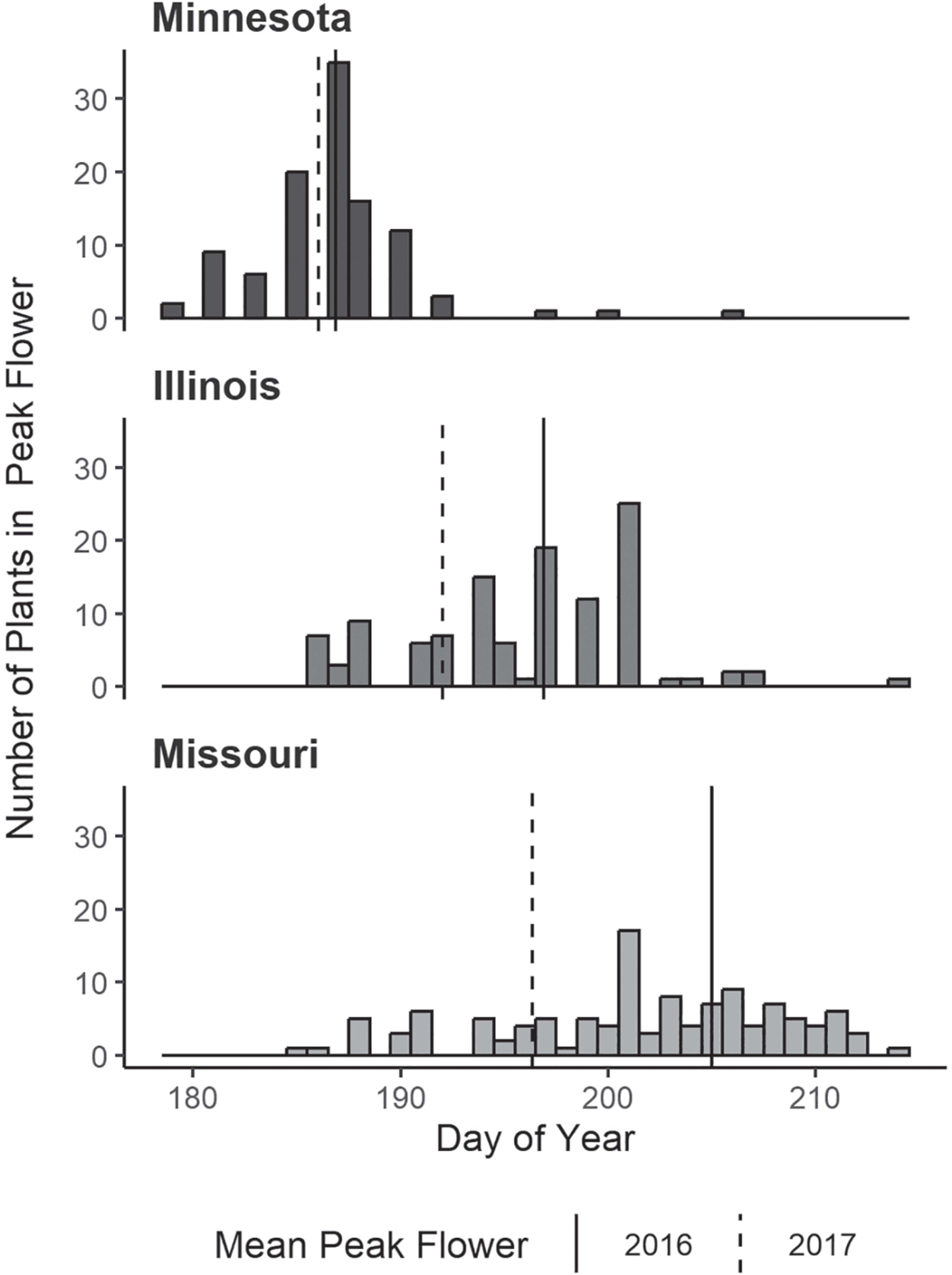
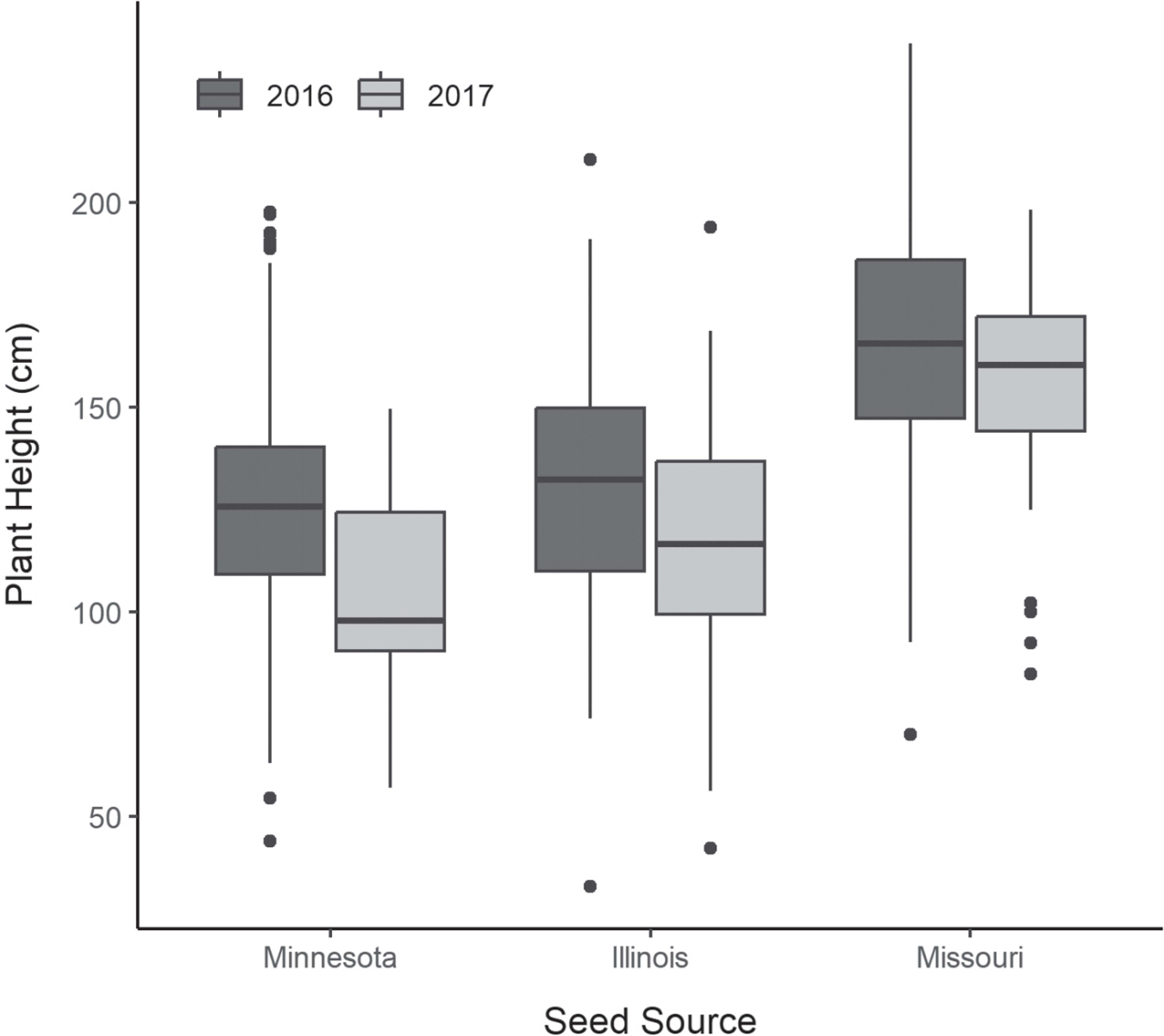
Averaged across both years, northern plants produced the most flowers (26.6, SD =22.6), 4.5 more than central plants and 14.7 more than southern plants. Flowering phenology was found to vary significantly by seed source for 2016 data (P<0.001) (Figure 3). A Tukey’s honest significant difference (HSD) test found all three seed sources varied significantly in mean day of peak flower (P≤0.047), with plants sourced from Minnesota (187, SD=4.2) achieving peak flower about 10 days earlier than those from Illinois (197, SD=5.4), and Illinois plants about eight days earlier than those from Missouri (205, SD=6.1). Flowering duration of individual plants did not significantly vary among seed sources (mean=18–21 days). Interannual variation in mean day of peak flower was considerably greater in plants from southern sources (8.63 days) than those from central sources (4.84 days) and northern sources (0.82 days).
Floral Visitation
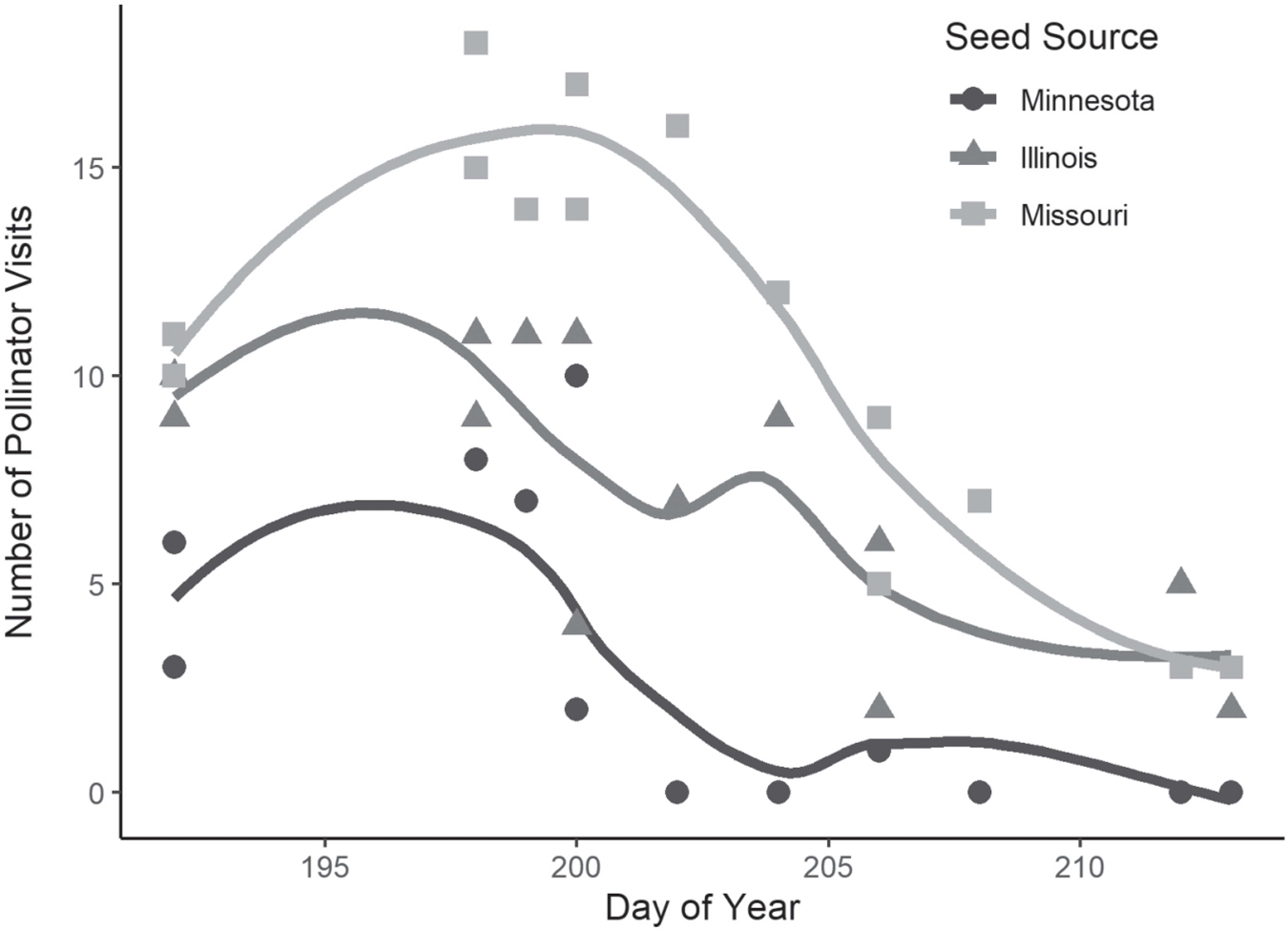
The number of floral visitors per observation period (15 min) ranged from zero to 18 (Figure 4). The number of visitors varied significantly by seed source and observation date (P<0.001), but not by time of day (morning or afternoon), reflecting sequential flowering phenology. On average, plants sourced from Missouri received the greatest number of floral visits per observation period (11, SD=5.0), followed by those sourced from Illinois (7.4, SD=3.3) and Minnesota (5.3, SE=3.1). Length of floral visitation ranged from one second to seven minutes and 21 seconds, with a mean of 54.8 seconds (SD=64.9). Analysis of variance found weak evidence (P=0.09) for variation in visit length among seed sources, with the longest visits for the Illinois (local) seed source (66.2 s, SD=81.5) followed by Minnesota (53.8 s, SD=67.4) and Missouri (47.9 s, SD=50.4).
Plant Height
The best fit linear model found that plant height varied significantly among seed source and year, with each predictor having an additive effect (P<0.001) (Figure 5). In both years the late-flowering plants from the Missouri seed source were the tallest of the three sources (mean=161, SD=31.0 cm), followed by those from the mid-flowering Illinois source (128, SD=30.3 cm), with the early-flowering plants from the Minnesota source exhibiting the shortest plant height (122, SD=31.1 cm). Tukey’s HSD test found the height of Missouri sourced plants was significantly greater than those from both Illinois and Minnesota sources (P<0.001), but plant height did not significantly differ between plants from Illinois and Minnesota (P=0.278). Plant height was significantly greater in 2016 than in 2017 (P<0.001). On average, plants sourced from Missouri were 10 cm taller, plants sourced from Illinois were 14 cm taller, and plants sourced from Minnesota were 26 cm taller in 2016 than in 2017.
Fruit Set and Abortion
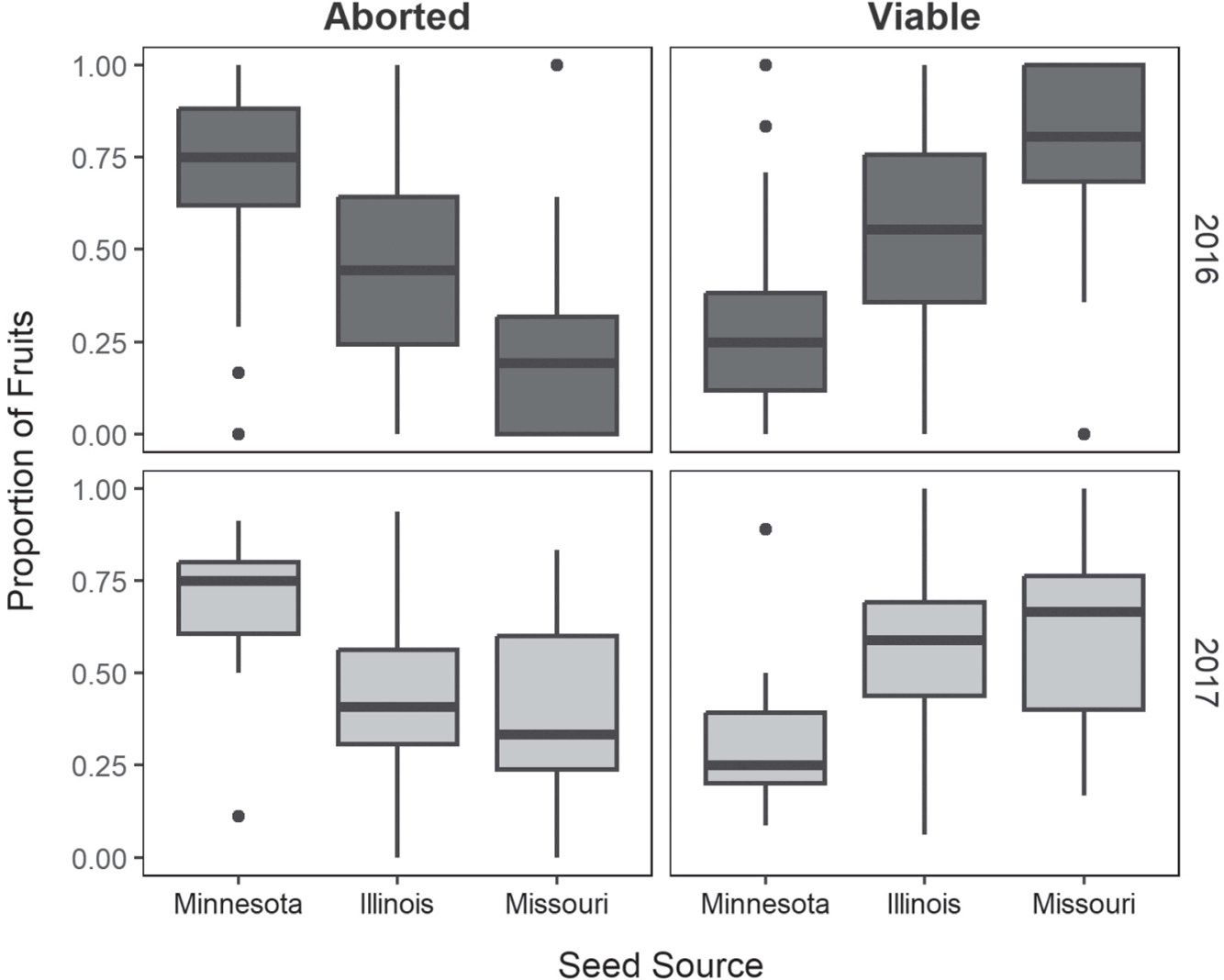
The minimal adequate model for viable fruit set was an additive generalized linear model including seed source and year (2016, 2017). Overall, the number of viable fruits varied among seed sources with plants from the Illinois source (mean=11.6, SD=12.2) producing significantly more than those from either Missouri (8.59, SD=7.87) or Minnesota (6.81, SD=7.27; P≤0.03) when summed across both years. However, the elevated fruit count of plants sourced from Illinois was overwhelmingly influenced by the first data collection year (2016; 12.7, SD=13.1). The minimal adequate model for fruit abortion was the maximal model, which includes an interaction term between seed source and year. The number of aborted fruits varied significantly among seed sources, with the greatest number observed for Minnesota plants (mean=19.8, SD=18.6) followed by Illinois (10.5, SD=11.9) and Missouri (3.32, SD=4.64; P <0.001). The minimal adequate model for the proportion of viable fruit retained a singular factor: population. All populations differed significantly from each other in the proportion of viable fruit (P <0.001), with Missouri having the highest proportion of viable fruit (0.73, SD=0.23), followed by Illinois (0.55, SD=0.25), and Minnesota (0.28, SD=0.20) (Figure 6).
DISCUSSION
Common garden performance over multiple years demonstrated significant variation among common milkweed seed sources in flowering phenology, floral visitation, height, and reproduction. In step with latitude and climate of the midwestern United States, northern plants from cooler climates with shorter growing seasons were shorter and flowered earlier than southern plants. Later flowering phenology was associated with increased floral visitation and may have driven the observed variation in fruit set. Specifically, southern plants flowered later and produced more viable fruits and fewer aborted fruits than northern plants, both in total count and relative proportion. These patterns appear to be the result of heritable genetic variation and not maternal effects, as they persisted over three years, including two resprout generations of the herbaceous, perennial plant. However, transgenerational phenotypic plasticity cannot be excluded as a contributor of the observed variation, as the maternal plants grew in disparate geographic and climatic environments (Herman and Sultan 2011).
Interestingly, persistent geographic variation is inconsistent with a recent study on range-wide variation in vegetative common milkweed traits and their effect on monarch larvae (DeLaMater et al. 2021). Biogeographic clines were found for all but three of 13 traits considered, but clines did not persist in a greenhouse common garden environment over 12 months and two resprout generations. The DeLaMater et al. (2021) study did not consider reproductive traits, but their field measurements also found northern plants to be shorter, as was observed in the outdoor common garden study presented here. An independent effort, the Milkweed Adaptation Research and Education Network (MAREN 2024) was founded in 2015 at St. Olaf College in Minnesota to investigate local adaptation in common milkweed. Their recent paper found evidence of a latitudinal cline in germination, with northern sources germinating faster and to a higher proportion (Mohl et al. 2023). Other MAREN studies have investigated plant-monarch interactions, finding a positive correlation between leaf number, source latitude (Rice et al. 2021), and presence of monarch eggs (Msuya et al. 2021). When taken together with the results from this study, potential divergent advantages for herbivores and nectarivores appear, as southern sources may provide floral visitors with a longer flowering period during which to nectar, but northern sources may produce more leaves, making them more attractive for monarch oviposition. These potential tradeoffs are especially impactful given that common milkweed supports not only monarchs but 112 unique insect species, including nine specialist herbivores (Wilhelm and Rericha 2017; Miles et al. 2022). Additional studies are still needed to elucidate the relative genetic and environmental contributions to clines in vegetative and reproductive traits in this widespread and ecologically significant species.
Previous work on common milkweed found the number of mature pods per stem is positively correlated with the number of inflorescences (umbels) per stem and the number of flowers per inflorescence (Willson and Rathcke 1974; Willson and Price 1977). This may be explained in part by a demonstrated positive correlation between floral display size and length of floral visits, which was found to positively impact female plant fitness in this species (La Rosa and Conner 2017). However, a coarse estimate of the relative number of flowers produced in this study found northern plants produced the most flowers on average, despite producing the fewest and lowest proportion of viable fruits of the three seed sources. It is important to note this estimate does not consider the number of umbels per stem or the size of umbels (number of flowers per inflorescence), which is known to impact pollinator visitation and viable fruit set (Willson and Price 1977).
Variation in flowering phenology within populations is known to impact fitness in this genus, with fruit set for late-flowering plants about twice that of early-flowering plants (Kephart 1987). Greater variation was observed among seed sources than within seed sources, difference in fruit set among seed sources were very similar to this finding, with about a twofold increase in viable fruit set between the earliest flowering seed source (Minnesota) and the middle flowering seed source (Illinois), and a threefold increase between the earliest flowering seed source and the latest flowering seed source (Missouri). Interestingly, the latitudinal cline in flowering phenology observed was consistent with a recent study of three perennial forb species in Minnesota where opposite impacts for fitness were found (Rushing et al. 2021). In addition to impacting reproductive fitness, variation in flowering phenology has implications for gene flow among individuals and populations as a result of greater or less flowering synchrony within and between populations (Rivest et al. 2021). Increased variation in intraspecific flowering phenology may benefit pollinators through greater temporal diversity in nectar resources, particularly in species-poor communities not benefiting from interspecific variation. However, decreased flowering synchrony can reduce cross-pollination, with implications for reproductive success, maintenance of genetic diversity, and evolutionary potential (Franklin and Frankham 1998; Richardson and Wagenius 2021).
Appropriate sourcing of plant material for ecological restoration and/or habitat creation is vital for the successful establishment and persistence of the targeted plant community, as well as ecological function (Gallagher and Wagenius 2015; Erickson and Halford 2020; Kettenring and Tarsa 2020). In the past decade awareness of the plight of the monarch butterfly, specifically the imperiled annual migration of the eastern North American population, has spurred swift and significant investment in the creation of monarch habitat throughout the summer breeding range (Lewandowski and Oberhauser 2016; Thogmartin et al. 2017a). Plant species composition of constructed or restored monarch habitat can vary but must include one or more species of milkweed (Asclepias spp.), the obligate larval host of the monarch butterfly, which also serve as important nectar plants for monarchs and other pollinators (Southwick 1983; Tillman and Carpenter 2014; Wilson 2021). It is also recommended plantings include a diversity of nectar-producing plants with bloom times spanning the entire growing season and migration period, from spring through fall (Havens and Vitt 2016). The findings of this study demonstrate the impact of seed sourcing on intraspecific variation in plant traits directly related to plant reproduction and pollinator resources, with southern sources flowering later and producing more viable fruits than northern sources. Interestingly, results indicate a mix of seed sources can increase the blooming period of a species in a single location by two to six times, or 30–50 days. Given the substantial clonal spread of common milkweed, as well as the numerous seeds contained in a single fruit, experimenting with increased variation in flowering phenology through mixing of seed sources may be a net positive for pollinators without any significant loss to species recruitment (Betz and Lamp1992; He and Agrawal 2020).
In addition to seed sourcing, these data must also be interpreted in the context of climate change. An analysis of flowering phenology across the native range of common milkweed found higher temperatures were correlated with symmetrical phenology shifts earlier (Howard 2018). Common milkweed plants moved to a cooler climate (e.g., seed from Missouri moved to the common garden in Illinois) fared well, but plants moved to a warmer climate (e.g., seed from Minnesota moved to the common garden in Illinois) demonstrated decreased reproduction 20–40% below that of plants sourced locally or from further south. These results suggest that reproductive capacity could decrease under climate change, potentially hampering population growth and the capacity to colonize new sites. Common milkweed produces wind-dispersed seeds, and plant height is expected to impact seed dispersal distance, with dispersal distance increasing with height of release (Morse and Schmitt 1985). Therefore, the impact of climate change on phenology and plant height at point of seed dispersal has implications for the number of seeds produced and their dispersal ability. The results indicate decreased performance under climate change, but simultaneously provide a potential mitigation strategy: the introduction of southern genotypes. To keep pace with rapid climate change, land management practitioners could introduce seeds from southern sources to naturally occurring or restored populations. Climate-informed sourcing is actively under investigation for a wide range of plant taxa (e.g., Jochems et al. 2022; St. Clair et al. 2022; Woolridge et al. 2023) with a segment of the restoration community trending towards the recommendation of mixing sources within a region (e.g., Bucharova et al. 2019; Hancock et al. 2023; Nolan et al. 2023). Given sufficient overlap in flowering times, interbreeding between local and non-local sources could introduce adaptive phenological traits and expedite adaptation to changing climates (Aitken and Whitlock 2013). However, studies of additional common milkweed populations across multiple field sites are necessary to determine the extent of phenological variation and the potential value of and risks associated with assisted gene flow to maintain population fitness under climate change.
In summary, wild-collected seed from three populations of common milkweed located across a 750 km latitudinal gradient in the midwestern United States demonstrated persistent phenological and phenotypic differences in a centrally located common garden over multiple growing seasons. Plants from northern sources flowered earlier, were shorter at maturity, and demonstrated considerably less interannual variation in phenology than those from southern sources. Plants from southern sources, which flowered later, received greater floral visitation, and produced more viable fruits than those from northern sources. These results suggest that plants from southern sources may perform better than those from local or northern sources under restoration settings. In addition, the results suggest populations of common milkweed may demonstrate decreased fruit production under climate change, with implications for population persistence and colonization of new sites. However, assisted gene flow through the introduction of seed from southern populations to higher latitudes could contribute novel adaptive phenological traits and expedite adaptation to changing climates. Additionally, these results suggest mixing of seed sources can greatly expand flowering windows in restored or constructed habitats with implications for pollinator conservation. Relatedly, mid-season mowing of milkweed has been shown to be a successful management strategy for supporting monarch reproduction because it serves as a phenological reset and adult monarchs prefer to oviposit on regenerating milkweed stems free from enemies (Fischer et al. 2015; Haan and Landis 2019; Knight et al. 2019). We encourage future studies to investigate how mixed-source milkweed restorations may provide valuable phenological variation without the need for mowing, which does pose risks for the invertebrate community.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization, K.H., and J.F.; seed collection, J.F. and K.H.; methodology, J.F. and K.H.; data collection, I.V. and J.F.; formal analysis, J.F.; data curation, J.F. and I.V.; writing—original draft preparation, J.F., I.V., and K.H.; writing—review and editing, J.F., I.V., and K.H.; visualization, J.F. and I.V.; supervision, K.H. and J.F.; All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
We would like to thank Ginger Allington, formerly of the Missouri Botanical Garden, and Ricky Garza, of the Minnesota Landscape Arboretum, for seed collection; the Chicago Botanic Garden Horticulture staff for propagation and garden maintenance, especially Jamie Berlin; Lake Forest College BIO 384 Plant Biology students for reproductive trait measurements (2015–2018); and interns Tia Chung-Swanson and Rachel Kreb for field data collection (2016). This work was supported in part by the Chicago Botanic Garden’s College First and REU Site Programs (under the 2017 NSF grant DBI-1461007). Lastly, we are grateful to the three anonymous reviewers who provided helpful comments on earlier drafts of this manuscript.
LITERATURE CITED
Aitken, S. N., and M. C. Whitlock. (2013). Assisted gene flow to facilitate local adaptation to climate change. Annual Review of Ecology, Evolution, and Systematics 44: 367–388.
Anderson, J. T., D. W. Inouye, A. M. McKinney, R. I. Colautti, and T. Mitchell-Olds. (2012). Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proceedings of the Royal Society B 279: 3843–3852.
Betz, R. F., and H. F. Lamp. (1992). Flower, pod, and seed production in eighteen species of milkweeds (Asclepias). Pp. 25–30 in Proceedings of the twelfth North American prairie conference: Recapturing a vanishing heritage, D. D. Smith and C. Jacobs, editors. University of Northern Iowa, Cedar Falls, Iowa.
Breed, M. F., P. A. Harrison, A. Bischoff, P. Durruty, N. J. C. Gellie, E. K. Gonzales, K. Havens, et al. (2018). Priority actions to improve provenance decision-making. BioScience 68: 510–516.
Broadhurst, L. M., A. Lowe, D. J. Coates, S. A. Cunningham, M. McDonald, P. A. Vesk, and C. Yates. (2008). Seed supply for broadscale restoration: maximizing evolutionary potential. Evolutionary Applications 1: 587–597.
Brower, L. P., O. R. Taylor, E. H. Williams, D. A. Slayback, R. R. Zubieta, and M. I. Ramírez. (2012). Decline of monarch butterflies overwintering in Mexico: Is the migratory phenomenon at risk? Insect Conservation and Diversity 5: 95–100.
Bucharova, A., M. Frenzel, K. Mody, M. Parepa, W. Durka, and O. Bossdorf. (2016). Plant ecotype affects interacting organisms across multiple trophic levels. Basic and Applied Ecology 17: 688–695.
Bucharova, A., O. Bossdorf, N. Hölzel, J. Kollman, R. Prasse, and W. Durka (2019). Mix and match: Regional admixture provenancing strikes a balance among different seed-sourcing strategies for ecological restoration. Conservation Genetics 20: 7–17
Bucharova, A., C. Lampei, M. Conrady, E. May, J. Matheja, M. Meyer, and D. Ott. (2021). Plant provenance affects pollinator network: Implications for ecological restoration. Journal of Applied Ecology 59: 373–383.
CBD. (2024). Convention on Biological Diversity. Decision adopted by the Conference of the Parties to the Convention on Biological Diversity on 1 November 2024. Available at www.cbd.int/doc/decisions/cop-16/cop-16-dec-20-en.pdf. (Accessed January 18, 2025)www.cbd.int/doc/decisions/cop-16/cop-16-dec-20-en.pdf
Crawley, M. J. (2015). Statistics: An introduction using R, Second Edition. Wiley, Imperial College London, UK.
DeLaMater, D. S., J. J. Couture, J. R. Puzey, and H. J. Dalgleish. (2021). Range-wide variations in common milkweed traits and their effect on monarch larvae. American Journal of Botany 108: 388–401.
Durant, J. M., D. Hjermann, T. Anker-Nilssen, G. Beaugrand, A. Mysterud, N. Pettorelli, and N. C. Stenseth. (2005). Timing and abundance as key mechanisms affecting trophic interactions in variable environments. Ecology Letters 8: 952–958.
Erickson, V. J., and A. Halford. (2020). Seed planning, sourcing, and procurement. Restoration Ecology 28: S219–S227.
Fick, S. E., and R. J. Hijmans, (2017). WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302-4315. Available at https://www.worldclim.org/data/worldclim21.html. (Accessed January 20, 2025)https://www.worldclim.org/data/worldclim21.html
Fischer, S. J., E. H. Williams, L. P. Brower, and P. A. Palmiotto. (2015). Enhancing monarch butterfly reproduction by mowing fields of common milkweed. American Midland Naturalist 173: 229–240.
Flockhart, D. T. T., J.-B. Pichancourt, D. R. Norris, and T. G. Martin. (2014). Unravelling the annual cycle in a migratory animal: Breeding-season habitat loss drives population declines of monarch butterflies. Journal of Animal Ecology 84: 155–165.
Forrest, J. R. K. (2014). Plant–pollinator interactions and phenological change: What can we learn about climate impacts from experiments and observations? Oikos 124: 4–13.
Franklin, I. R., and R. Frankham. (1998). How large must populations be to retain evolutionary potential? Animal Conservation 1: 69–70.
Gaertner, E. A. (1979). The history and use of milkweed (Asclepias syriaca L.). Economic Botany 33: 119–123.
Gallagher, M. K., and S. Wagenius. (2015). Seed source impacts germination and early establishment of dominant grasses in prairie restorations. Journal of Applied Ecology 53: 251–263.
Gehring, C. A., C. M. Sthultz, L. Flores-Rentería, A. V. Whipple, and T. G. Whitham. (2017). Tree genetics defines fungal partner communities that may confer drought tolerance. Proceedings of the National Academy of Science of the United States of America 114: 11169–11174.
Genes, L., and R. Dirzo. (2022). Restoration of plant-animal interactions in terrestrial ecosystems. Biological Conservation 265: 109393.
Gosney, B., J. O’Reilly-Wapstra, L. Forster, C. Whiteley, and B. Potts. (2017). The extended com-munity-level effects of genetic variation in foliar wax chemistry in the forest tree eucalyptus globulus. Journal of Chemical Ecology 43: 532–542.
Haan, N. L. and D. A. Landis. (2019). Grassland disturbance increases monarch butterfly oviposition and decreases arthropod predator abundance. Biological Conservation 233: 185–192.
Hancock, N. M., F. Encinas-Viso, and L. M. Broadhurst. (2023). A documented paradigm shift in seed sourcing: attitudinal changes to using local native seed for ecological restoration. Restoration Ecology 31: e13845.
Hartzler, R. G., and D. D. Buhler. (2000). Occurrence of common milkweed (Asclepias syriaca) in cropland and adjacent areas. Crop Protection 19: 363–366.
Havens, K., and P. Vitt. (2016). The importance of phenological diversity in seed mixes for pollinator restoration. Natural Areas Journal 36: 531–537.
Havens, K., P. Vitt, S. Still, A. T. Kramer, J. B. Fant, and K. Schatz. (2015). Seed sourcing for restoration in an era of climate change. Natural Areas Journal 35: 122–133.
He, E., and A. A. Agrawal. (2020). Clonal versus non-clonal milkweeds (Asclepias spp.) respond differently to stem damage, affecting oviposition by monarch butterflies. PeerJ 8: e10296.
Hereford, J. (2009). A quantitative survey of local adaptation and fitness trade-offs. The American Naturalist 173: 579–588.
Herman, J. J., and S. E. Sultan. (2011). Adaptive transgenerational plasticity in plants: Case studies, mechanisms, and implications for natural populations. Frontiers in Plant Sciences 2: 102.
Hobbs, R. J., and V. A. Cramer. (2008). Restoration ecology: Interventionist approaches for restoring and maintaining ecosystem function in the face of rapid environmental change. Annual Review of Environment and Resources 33: 39–61.
Hothorn, T., F. Bretz, P. Westfall, R. M. Heiberger, A. Schuetzenmeister, and S. Scheib. (2017). Package “multcomp” - Simultaneous inference in general parametric models. Available at https://doi.org/https://cran.r-project.org/web/packages/multcomp/multcomp.pdf. (Accessed July 18, 2024).https://doi.org/https://cran.r-project.org/web/packages/multcomp/multcomp.pdf
Howard, A. F. (2018). Asclepias syriaca (common milkweed) flowering date shift in response to climate change. Scientific Reports 8: 17802.
IUCN. (2020). The Bonn Challenge. Available at https://www.bonnchallenge.org/. (Accessed January 18, 2025).https://www.bonnchallenge.org/
Jochems, L. W., J. A. Lau, L. A. Brudvig, and E. Grman. 2022. Do southern seed or soil microbes mitigate the effects of warming on establishing prairie plant communities? Ecological Applications 32: e02487.
Johnson, G., F. C. Sorensen, J. B. S. Clair, and R. C. Cronn. (2004). Pacific Northwest forest tree seed zones. Native Plants Journal 5: 131–140.
Kettenring, K. M., and E. E. Tarsa. (2020). Need to seed? Ecological, genetic, and evolutionary keys to seed-based wetland restoration. Frontiers in Environmental Science 8: 109.
Kephart, S. R. (1987). Phenological variation in flowering and fruiting of Asclepias. American Midland Naturalist 118: 64–76.
Knight, S. M., D. R. Norris, R. Derbyshire, and D. T. T. Flockhart. (2019). Strategic mowing of roadside milkweeds increases monarch butterfly oviposition. Global Ecology and Conservation 19: e00678.
Kramer, A. T.. and K. Havens. (2009). Plant conservation genetics in a changing world. Trends in Plant Science 14: 599–607.
La Rosa, R. J., and J. K. Conner. (2017). Floral function: Effects of traits on pollinators, male and female pollination success, and female fitness across three species of milkweeds (Asclepias). American Journal of Botany 104(1): 150–160.
Leimu, R.. and M. Fischer. (2008). A meta-analysis of local adaptation in plants. PLoS One 3: e4010.
Lewandowski, E. J., and K. S. Oberhauser. (2016). Contributions of citizen scientists and habitat vol-unteers to monarch butterfly conservation. Human Dimensions of Wildlife 22: 55–70.
Malcolm, S. B. (1994). Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology 5: 101–117.
MAREN. (2024). Milkweed adaptation research and education network. Available at https://pages.stolaf.edu/milkweed/ (Accessed January 22, 2025)https://pages.stolaf.edu/milkweed/
McKay, J. K., C. E. Christian, S. Harrison, and K. J. Rice. (2005). “How Local Is Local?”—A review of practical and conceptual issues in the genetics of restoration. Restoration Ecology 13: 432-440.
Memmot, J., P. G. Craze, N. M. Waser, and M. V. Price. (2007). Global warming and the disruption of plant–pollinator interactions. Ecology Letters 10: 710–717.
Midwest Association of Fish and Wildlife Agencies. (2018). Mid-America monarch conservation strategy, 2018–2038, Version 1.0. Available at https://www.mafwa.org/wp-content/up-loads/2018/07/MAMCS_June2018_Final.pdf. (Accessed July 18, 2024).https://www.mafwa.org/wp-content/up-loads/2018/07/MAMCS_June2018_Final.pdf
Miles, L. S., D. Murray-Stoker, V. J. Nhan, and M. T. J. Johnson. (2022). Effects of urbanization on specialist insect communities of milkweed are mediated by spatial and temporal variation. Ecosphere 13: e4222.
Mohl, E. K., A. C. McCall, M. Wood, L. Sherman, M. V. Reid, P. A. Saunders, S. E. Scanga, et al. (2023). Common milkweed seeds exhibit latitudinal clines more consistent with adaptation to growing season length than temperature. Restoration Ecology 31: e13878.
Morse, D. H., and J. Schmitt. (1985). Propagule size, dispersal ability, and seedling performance in Asclepias syriaca. Oecologia 67: 372–379.
Mortlock, B. W. (2000). Local seed for revegetation. Ecological Management & Restoration 1: 93–101.
Msuya, K., L. Sherman, S. Ronneberg, S. Dlamini, and E. Mohl. (2021). Colonization trends in common milkweed, Asclepias syriaca, in central Minnesota. CURI Program Fall Showcase, St. Olaf College. Available at: https://pages.stolaf.edu/milkweed/wp-content/uploads/sites/1333/2022/03/MSUYA.SHERMAN.RONNEBERG.DLAMINI.MOHLCURI2021POSTER.pdf. (Accessed January 22, 2025).https://pages.stolaf.edu/milkweed/wp-content/uploads/sites/1333/2022/03/MSUYA.SHERMAN.RONNEBERG.DLAMINI.MOHLCURI2021POSTER.pdf
NOAA. (2019). National Oceanic and Atmospheric Administration, National Centers for Environmental Information, Climate Data Online. Station: Chicago Botanic Garden, IL US (GHCND:USC00111497). Available at: https://www.ncdc.noaa.gov/cdo-web/datasets/GHCND/stations/GHCND:USC00111497/detail. (Accessed January 20, 2025).https://www.ncdc.noaa.gov/cdo-web/datasets/GHCND/stations/GHCND:USC00111497/detail
Nolan, M.P., J. C. Luong, J. M. Valliere, S. J. Mazer, and C. M. D’Antonio. (2023). Rethinking local seed sourcing for the restoration of a foundational grass species in California. Restoration Ecology 31: e13992.
Peralta, G., D. P. Vázquez, N. P. Chacoff, S. B. Lomáscolo, G. L. W. Perry, and J. M. Tylianakis. (2020). Trait matching and phenological overlap increase the spatio-temporal stability and functionality of plant–pollinator interactions. Ecology Letters 23: 1107–1116.
Piao, S., Q. Liu, A. Chen, I. A. Janssens, Y. Fu, J. Dai, L. Liu, et al. (2019). Plant phenology and global climate change: Current progresses and challenges. Global Change Biology 25: 1922–1940.
Pleasants, J. (2017). Milkweed restoration in the Midwest for monarch butterfly recovery: Estimates of milkweeds lost, milkweeds remaining and milkweeds that must be added to increase the monarch population. Insect Conservation and Diversity 10: 42–53.
Prober, S. M., M. Byrne, E. H. McLean, D. A. Steane, B. M. Potts, R. E. Vaillancourt, and W. D. Stock. (2015). Climate-adjusted provenancing: a strategy for climate-resilient ecological restoration. Frontiers in Ecology and Evolution 3: 65.
R Core Team. (2021). R: A language and environment for statistical computing. Available at https://www.R-project.org/. (Accessed July 18, 2024).https://www.R-project.org/
Rice, S., C. Wilkens, M. Wood, and E. Mohl. (2021). Evidence of geographic local adaptation in fitness and growth traits of Asclepias syriaca. CURI Program Fall Showcase, St. Olaf College. Available at: https://pages.stolaf.edu/milkweed/wp-content/uploads/sites/1333/2022/03/RICE-WILKENS-WOOD-MOHL-CURI-2021-Poster-1-compressed.pdf. (Accessed January 22, 2025).https://pages.stolaf.edu/milkweed/wp-content/uploads/sites/1333/2022/03/RICE-WILKENS-WOOD-MOHL-CURI-2021-Poster-1-compressed.pdf
Richardson, L. K., and S. Wagenius. (2021). Fire influences reproductive outcomes by modifying flowering phenology and mate-availability. New Phytologist 233: 2083–2093.
Rivest, S., G. Lajoie, D. A. Watts, and M. Velland. (2021). Earlier spring reduces potential for gene flow via reduced flowering synchrony across an elevational gradient. American Journal of Botany 108: 538–545.
Rushing, N. S., S. A. Flint, and R. G. Shaw. (2021). Latitude of seed source impacts flowering phenology and fitness in translocated plant populations. Restoration Ecology 29: e13464.
Seiber, J. N., L. P. Brower, S. M. Lee, M. M. McChesney, H. T. A. Cheung, C. J. Nelson, and T. R. Watson. (1986). Cardenolide connection between overwintering monarch butterflies from Mexico and their larval food plant, Asclepias syriaca. Journal of Chemical Ecology 12: 1157–1170.
Semmens, B. X., D. J. Semmens, W. E. Thogmartin, R. Wiederholt, L. López-Hoffman, J. E. Diffendorfer, J. M. Pleasants, et al. (2016). Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Scientific Reports 6: 1–7.
Society for Ecological Restoration International Science & Policy Working Group. (2004). The SER International Primer on Ecological Restoration. Available at https://www.ctahr.hawaii.edu/lit-tonc/PDFs/682_SERPrimer.pdf. (Accessed July 18, 2024).https://www.ctahr.hawaii.edu/lit-tonc/PDFs/682_SERPrimer.pdf
Southwick, E. E. (1983). Nectar biology and nectar feeders of common milkweed, Asclepias syriaca L. Journal of the Torrey Botanical Society 110: 324–334.
St.Clair, J. B., B. A. Richardson, N. Stevenson-Molnar, G. T. Howe, A. D. Bower, V. J. Erickson, B. Ward, et al. (2022). Seedlot selection tool and climate-smart restoration tool: Web-based tools for sourcing seed adapted to future climates. Ecosphere 13: e4089.
Stephenson, A. G. (1981). Flower and fruit abortion: Proximate causes and ultimate functions. Annual Review of Ecology, Evolution, and Systematics 12: 253–279.
Thogmartin, W. E., L. López-Hoffman, J. Rohweder, J. Diffendorfer, R. Drum, D. Semmens, S. Black, et al. (2017a). Restoring monarch butterfly habitat in the midwestern US: “All hands on deck.” Environmental Research Letters 12: 074005.
Thogmartin, W. E., R. Wiederholt, K. Oberhauser, R. G. Drum, J. E. Diffendorfer, S. Altizer, O. R. Taylor, et al. (2017b). Monarch butterfly population decline in North America: Identifying the threatening processes. Royal Society Open Science 4: 170760.
Tillman, P. G., and J. E. Carpenter. (2014). Milkweed (Gentianales: Apocynaceae): A farmscape resource for increasing parasitism of stink bugs (Hemiptera: Pentatomidae) and providing nectar to insect pollinators and monarch butterflies. Environmental Entomology 43: 370–376.
Trudeau, J., B. Obama, and E. Peña. (2016). Leaders’ statement on a North American climate, clean energy, and environment partnership. Available at https://www.pm.gc.ca/en/news/state-ments/2016/06/29/leaders-statement-north-american-climate-clean-energy-and-environment. (Accessed July 18, 2024).https://www.pm.gc.ca/en/news/state-ments/2016/06/29/leaders-statement-north-american-climate-clean-energy-and-environment
UN. (2015). Transforming our world: The 2030 agenda for sustainable development. Available at https://sdgs.un.org/publications/transforming-our-world-2030-agenda-sustainable-development-17981. (Accessed January 18, 2025).https://sdgs.un.org/publications/transforming-our-world-2030-agenda-sustainable-development-17981
UNEP. (2024). United Nations Decade on Ecosystem Restoration 2021–2030. Available at https://www.decadeonrestoration.org/. (Accessed January 22, 2025).https://www.decadeonrestoration.org/
USDOI. (2024). U.S. Department of the Interior. America the beautiful. Available at https://www.doi.gov/priorities/america-the-beautiful. (Accessed January 19, 2025).https://www.doi.gov/priorities/america-the-beautiful
Wilhelm, G., and L. Rericha. (2017). Flora of the Chicago region: A floristic and ecological synthesis. Indiana Academy of Science, Indianapolis.
Willson, M. F., and P. W. Price. (1977). The evolution of inflorescence size in Asclepias (Asclepiadaceae). Evolution 31: 495.
Willson, M. F and B. J. Rathcke. (1974). Adaptive design of the floral display in Asclepias syriaca L. American Midland Naturalist 92: 47.
Wilson, J. S. (2021). What North American bees are associated with milkweed (Asclepias) flowers? Western North American Naturalist 81: 171–180.
Woolridge, C. B., J. B. Fant, A. I. Flores, K. Schultz, and A. T. Kramer. (2023). Variation in overall fitness due to seed source: Projections for predictive provenancing. Restoration Ecology 31: e13717.