Introduction
Urothelial carcinoma (UC) accounted for >90% of all 549,393 new cases of bladder cancer worldwide in 2018. Most cases of UC are superficial, with about 25% being muscle invasive and 11% being locally advanced or metastatic at diagnosis (la/mUC). La/mUC has a poor prognosis, with a <15% 5-year survival rate. Metastatic urothelial carcinoma (mUC) has a 4.6% 5-year survival rate in the United States.1 A recent literature review, however, found that there is a significant lack of up-to-date epidemiological and treatment data on UC.1 First-line treatment of patients with mUC is currently platinum-based combination chemotherapy, including gemcitabine and cisplatin or methotrexate, vinblastine, and doxorubicin. These therapies have demonstrated poor overall survival.2 Immunotherapy options include pembrolizumab, atezolizumab, and avelumab.3 Patients whose disease is refractory to these therapies have had few options in the past, all of which are not curative, have low overall response rates, or have short durations of response. Examples include taxanes and erdafitinib, used in about 20% of patients with UC who have FGFR3 or FGFR2 mutations.3 Enfortumab vedotin (EV) is one of several novel, recently FDA-approved monoclonal antibodies for mUC refractory to both programmed cell death protein 1 (PD-1) or PD-L1 inhibitors and platinum-containing chemotherapies, and EV offers higher and longer response rates than the aforementioned alternative treatments. EV targets solid tumors expressing Nectin-4 and induces apoptotic activity via an attached microtubule disrupting agent, monomethyl auristatin E (MMAE).4–6 Nectin-4 is expressed in 97% of urothelial tumors and in weak to moderate levels in skin keratinocytes and skin appendages.5
In a Phase I study, EV administered at 1.25 mg/kg for 30 minutes on days 1, 8, and 15 of 28-day cycles was well tolerated and demonstrated a 42% response rate in patients with mUC who were ineligible for cisplatin or who experienced disease progression despite standard treatment with chemotherapy and immune checkpoint inhibitors.4,5 This is the typical treatment regimen with EV. In Phase II studies, EV demonstrated a 44% overall response rate in these patients.5 In Phase III trials, overall survival was greater in patients treated with EV compared with chemotherapy by about 4 months, and progression-free survival was longer by about 2 months. Adverse event rates were similar in both groups.2 Adverse events in response to EV included hyperglycemia, peripheral neuropathy, ocular disorders, cutaneous reactions, infusion site extravasations, and embryo-fetal toxicity.2,3 Although cutaneous toxicity was reported in 48% of patients in Phase II studies, the morphology of these eruptions remains poorly described.5 Previous reported descriptions of eruptions to EV have included a case of erythematous, scaly pink papules on the trunk and extremities; a case of intertriginous and flexural exanthema; one of an eczematoid and lichenoid rash with epidermal necrosis; and one of toxic epidermal necrolysis (TEN). Topical corticosteroids have been reported as a successful treatment strategy in all cases except TEN.7–12
Toxic erythema of chemotherapy (TEC) refers to various non-allergic cutaneous reactions in patients receiving chemotherapy.8 Often misdiagnosed as hypersensitivity allergic reactions, TEC can present with paresthesias; pruritus; and painful erythema and edema in the extremities, intertriginous areas, elbows, knees, and ears, usually 2 to 3 weeks after the onset of therapy. Histologic features include eccrine squamous syringometaplasia,* keratinocyte dysmaturation, apoptosis, necrosis, and vacuolar degeneration. Though TEC is often self-limiting, symptomatic therapies are frequently employed. Common first-line therapies include analgesics and topical corticosteroids. Persistent TEC is treated via systemic corticosteroids, chemotherapy dose reduction, or ultimately discontinuation of the offending agent.8 Here, we report a patient with two distinct cutaneous morphologies during treatment with EV for mUC that can both be classified histologically as TEC. To our knowledge, these hyperkeratotic rashes are the first described EV-related TEC eruption. We also propose that EV-related TEC eruptions may be polymorphous across patients and within the same patient.
Case Report
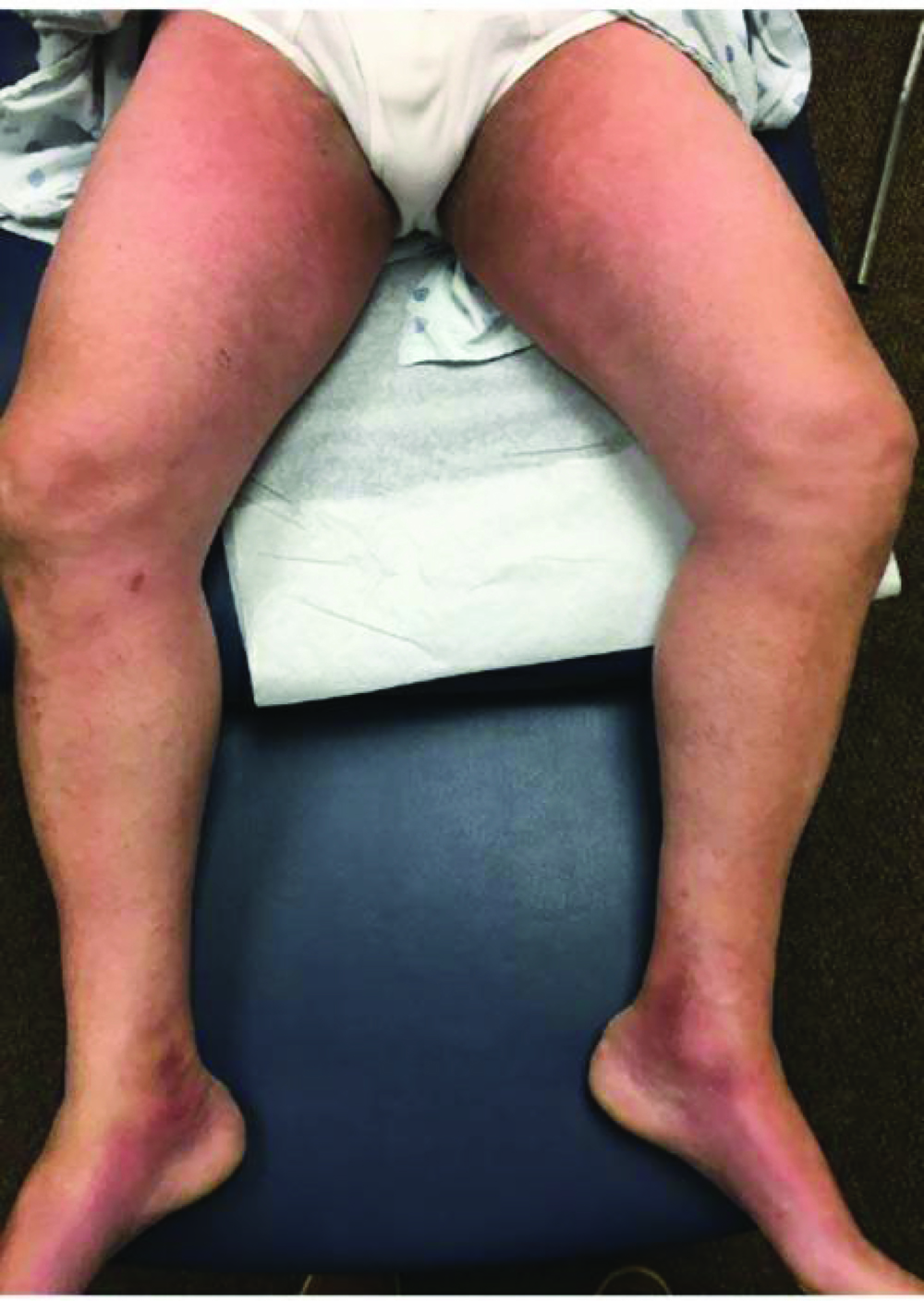
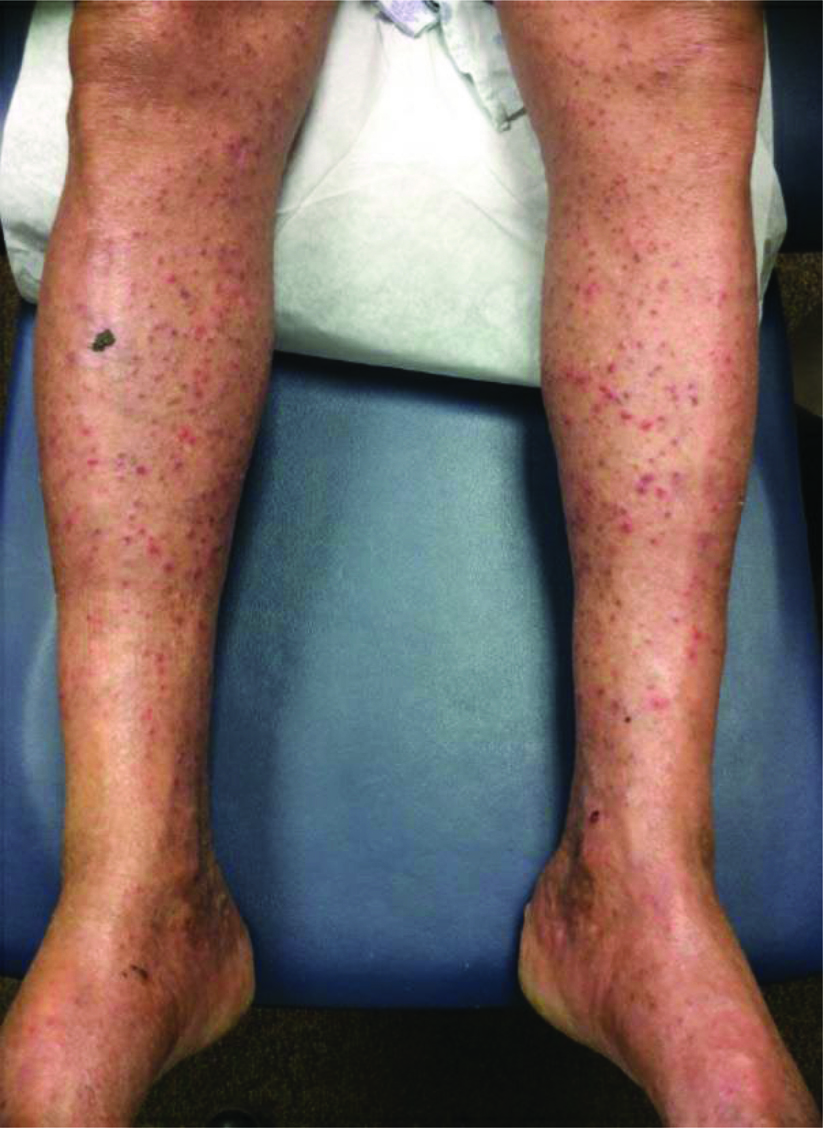
A 60-year-old Caucasian male with chemo- and immunotherapy refractory high-grade diffuse mUC was enrolled in a Phase III trial for salvage EV. His past medical history was significant for severe malnutrition, acute kidney injury (AKI), no known allergies, and brain metastasis of his mUC. After completing the standard regimen of 3 infusions at 1.25 mg/kg of EV over 15 days in his first cycle, the patient presented with burning, erythematous-to-violaceous, slightly edematous thin plaques on the palmoplantar and flexural skin (Figure 1). He also had ecchymotic changes on his feet, axillae, and groin and noted pain, warmth, and swelling in his lower extremities. Punch biopsy revealed epidermal dysmaturation and eccrine squamous syringometaplasia with mild vacuolar interface dermatitis,* consistent with TEC. The rash did not respond to 0.1% hydrocortisone but promptly resolved with topical triamcinolone 0.1% ointment. Four months later during cycle 6, day 8 of EV therapy, he developed diffuse, pruritic, erythematous-to-violaceous, non-folliculocentric, hyperkeratotic papules, with a few scattered tense vesicles more pronounced in the extremities compared with his trunk (Figure 2).
Repeat punch biopsy revealed vacuolar interface dermatitis with central epidermal necrosis and ulceration, epidermal dysmaturation, and superficial perivascular infiltrate, again consistent with TEC. Direct immunofluorescence was performed given the vesicles seen in the second eruption, but the negative result argued against an immunobullous eruption. This rash responded more slowly to topical triamcinolone 0.1% ointment, and the patient was given prednisone 1 mg/kg/day. His rash resolved in 3 weeks, leaving hyperpigmentation in affected areas. There was no appreciable nail or mucous membrane involvement. Thorough chart review excluded other potential causative medications.
Discussion
To our knowledge, this is the first report of TEC related to EV therapy. Past reports of cutaneous eruptions have described erythematous, scaly papules on the trunk and extremities; intertriginous and flexural exanthema; eczematoid and lichenoid rashes with epidermal necrosis; and TEN. The body regions affected in these cases overlap in part with the areas affected in our patient, notably the trunk, intertriginous, and flexural areas. Our patient’s presentation was more widespread, also affecting areas such as the entire upper and lower extremities. It also differed in its dermatologic description; we observed palmoplantar and flexural erythrodysesthesia* in his first presentation and widespread erythematous and hyperkeratotic papulovesicular eruption in his second presentation. Interestingly, while our patient’s first presentation on his distal upper and lower extremities and groin was classic for TEC, his second presentation was less so, given the location of the rash on his abdomen and unusual histologic presentation. Nonetheless, TEC can present with a broad spectrum of symptoms that make it often difficult to recognize. If misdiagnosed or untreated, its potential complications include continued exposure to the causative agent and extensive desquamation and post-inflammatory hyperpigmentation.
EV remains a critical component of mUC therapy given its higher and longer response rates compared with other options available to patients refractory to first-line treatments. Given its novelty, however, the specifics of its cutaneous toxicity are still unclear, making documenting and diagnosing them critical for both patient prognosis and for avoiding potentially harmful treatments. The expression of Nectin-4 in urothelial cancers and skin keratinocytes and appendages, along with previous reports of skin toxicity as a result of various antibody-drug conjugates, offers a possible explanation for both the therapeutic target in medications such as brentuximab vedotin and the cutaneous toxicity seen with EV therapy.6
The potential for TEC to be a polymorphous cutaneous reaction to EV highlights a need for dermatologist involvement in the healthcare team caring for patients with mUC to assist with diagnosis and management. Further detailed documentation of cases of cutaneous eruptions to EV, including both clinical and histologic images, will be critical toward aiding with this goal. Cutaneous eruptions in patients treated with EV should be referred to dermatology for appropriate workup, including, when indicated, biopsy for histopathologic confirmation.
Notes
- Eccrine squamous syringometaplasia: A benign, rare cutaneous reaction involving noninflammatory metaplasia of the cuboidal epithelial cells of the sweat glands in response to chemotherapy. ⮭
- Vacuolar interface dermatitis: Also known as liquefactive degeneration, this refers to vacuole formation at the dermal and epidermal junction, with lymphocytic infiltration in both layers. ⮭
- Erythrodysesthesia: Also known as hand-foot syndrome (HFS), this refers to symmetrical erythema, edema, tingling, and pain primarily on the palms of the hands and soles of the feet. ⮭
References
1. Hepp Z, Shah SN, Smoyer K, Vadagam P. Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis. J Manag Care Spec Pharm. 2021; 27(2):240–255. doi:10.18553/jmcp.2020.2028510.18553/jmcp.2020.20285
2. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021; 384(12):1125–1135. doi:10.1056/NEJMoa203580710.1056/NEJMoa2035807
3. Chang E, Weinstock C, Zhang L, et al. FDA approval summary: enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. 2021; 27(4):922–927. doi:10.1158/1078-0432.CCR-20-227510.1158/1078-0432.CCR-20-2275
4. Hanna KS. Updates and novel treatments in urothelial carcinoma. J Oncol Pharm Pract. 2019; 25(3):648–656. doi:10.1177/107815521880514110.1177/1078155218805141
5. Petrylak DP, Balar AV, O’Donnell PH, et al. EV-201: results of enfortumab vedotin monotherapy for locally advanced or metastatic urothelial cancer previously treated with platinum and immune checkpoint inhibitors. J Clin Oncol. 2019; 37(18_suppl):4505–4505.
6. Rosenberg JE, Heath E, Perez R, et al. Interim analysis of a phase I dose escalation trial of ASG-22CE (ASG-22ME; enfortumab vedotin), an antibody drug conjugate (ADC), in patients (Pts) with metastatic urothelial cancer (mUC). Ann Oncol. 2016; 27(suppl_6):788. doi:10.1093/annonc/mdw373.1610.1093/annonc/mdw373.16
7. Wu S, Adamson AS. Cutaneous toxicity associated with enfortumab vedotin treatment of metastatic urothelial carcinoma. Dermatol Online J. 2019; 25(2):6.
8. Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008; 59(3):524–529. doi:10.1016/j.jaad.2008.05.01810.1016/j.jaad.2008.05.018
9. Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting Nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016; 76(10):3003–3013. doi:10.1158/0008-5472.CAN-15-131310.1158/0008-5472.CAN-15-1313
10. Francis A, Jimenez A, Sundaresan S, Kelly B. A rare presentation of enfortumab vedotin–induced toxic epidermal necrolysis. JAAD Case Rep. 2021; 7:57–59. doi:10.1016/j.jdcr.2020.10.02010.1016/j.jdcr.2020.10.020
11. Sasaki R, Fujimura T, Lyu C, Aiba S. Severe eczematoid and lichenoid eruption with full-thickness epidermal necrosis developing from metastatic urothelial cancer treated with enfortumab vedotin. J Dermatol. 2020; 47(12):1436–1438. doi:10.1111/1346-8138.1557710.1111/1346-8138.15577
12. Keerty D, Graham L, Haynes E, Hembree TN. Flexural exanthema from enfortumab vedotin. Cureus. 2020; 12(5):e8102. doi:10.7759/cureus.810210.7759/cureus.8102