Introduction
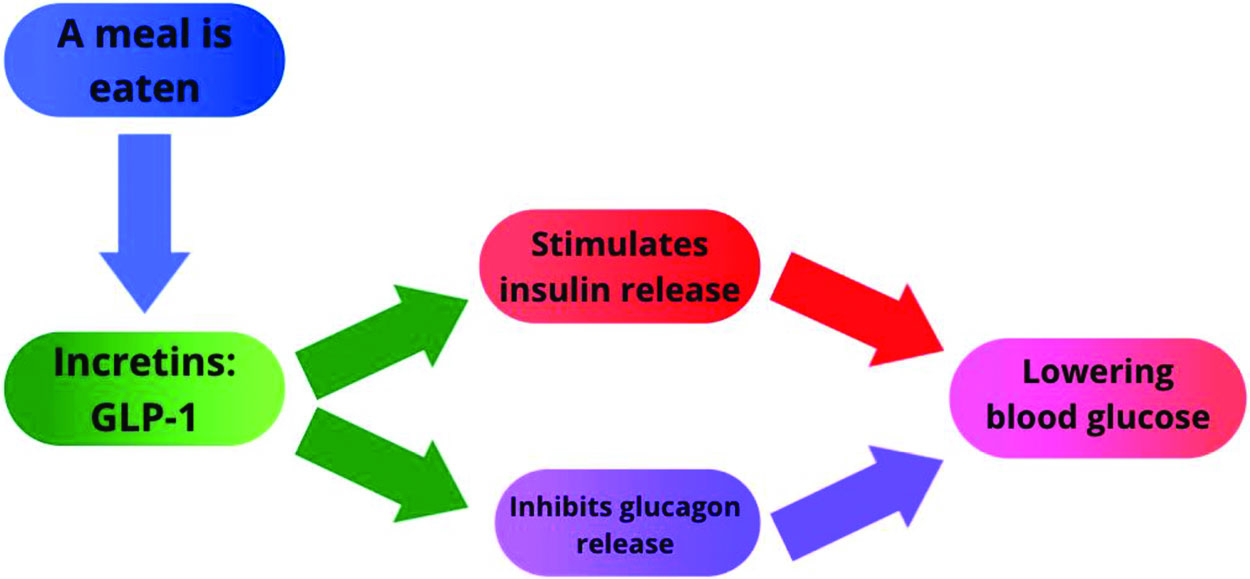
GLP-1 (Glucagon-Like Peptide 1) is an incretin mostly secreted by intestinal epithelial endocrine L-cells lining the inside of the large intestine and the α-cells of the pancreatic islets (Whalley et al., 2011). It is secreted into the bloodstream in response to nutrients. GLP-1 travels through the bloodstream to influence many different organs in our body such as the brain, liver, and pancreas (Schematically represented in Figure 1) (Alejandro et al., 2014; D’Alessio et al., 1994; Drucker & Nauck, 2006; Smith et al., 2014). GLP-1 binds to a G protein-coupled receptor (GPCR) on the cell membrane.
The pancreas is composed of two main compartments: the exocrine tissue (acinar cells and ducts), which represent about 80% of the pancreas, and endocrine tissue, highly vascularized and regrouped into islets of Langerhans embedded within the acinar cells, representing 2–3% of the pancreas. The exocrine cells secrete digestive enzymes, and the endocrine glands secrete hormones into the bloodstream, mostly to regulate blood glucose levels. There are five types of pancreatic islet cells: α-cells secreting glucagon, β-cells secreting insulin, δ-cells secreting somatostatin, PP cells secreting Pancreatic Polypeptide, and ε-cells secreting Ghrelin.
In the adult, GLP-1 is known to stimulate insulin secretion, inhibit glucagon release, and has a role in β-cell survival, targeting pathways known to regulate pancreatic development (Alejandro et al., 2014; Cras-Méneur et al., 2016; Cras-Méneur et al., 2002; Elghazi et al., 2017; Elghazi et al., 2017; Elghazi et al., 2002; Light et al., 2002; Talchai et al., 2015; Thorens et al., 1996; Villanueva-Peñacarrillo et al., 2001). This shifts the body to anabolic processes and lowers blood glucose.
Diabetes is a chronic health condition that affects how the body turns food into energy. There are two different types of diabetes. Type 1 occurs when the body’s immune cells attack the β-cells in the pancreas. This can destroy all the β-cells causing the pancreas to be unable to secrete any insulin or deplete the amount of β-cells causing the inability to produce sufficient amounts of insulin in response to a meal. Type 2 diabetes results when the body is not able to provide enough insulin to adequately lower blood glucose. Typically, type 2 diabetics have become insulin resistant, and their β-cells fail to adapt to the increased demand for insulin. GLP-1 has been used as a pharmacologic treatment for Type 2 diabetes because of its ability to increase insulin secretion and limit β-cell death. (Alejandro et al., 2014; Cras-Méneur et al., 2016; Cras-Méneur et al., 2002; Elghazi et al., 2017; Elghazi et al., 2017; Elghazi et al., 2002; Light et al., 2002; Talchai et al., 2015; Thorens et al., 1996; Villaneuva-Peñacarrillo et al., 2001).
While the effects of GLP-1 in adults have been extensively studied, few studies have addressed its impact on pancreatic development (Elghazi et al., 2017; Elghazi et al., 2017; Elghazi et al., 2002; Gromada et al., 1997; Light et al., 2002; Millamn et al., 2016; Pagliuca et al., 2014; Schindelin et al., 2012; Smith et al., 2014; Sun, X, & Kaufman, 2018; Talchai et al., 2015; Thorens et al., 1996; Villaneuva-Peñacarrillo et al., 2001). This project aims to investigate whether GLP-1 could affect the developing islets in the pancreas during embryonic development. To target GLP-1 signaling during development, we used mice that have a deletion of the GLP-1 receptor and are therefore insensitive to GLP-1 itself.
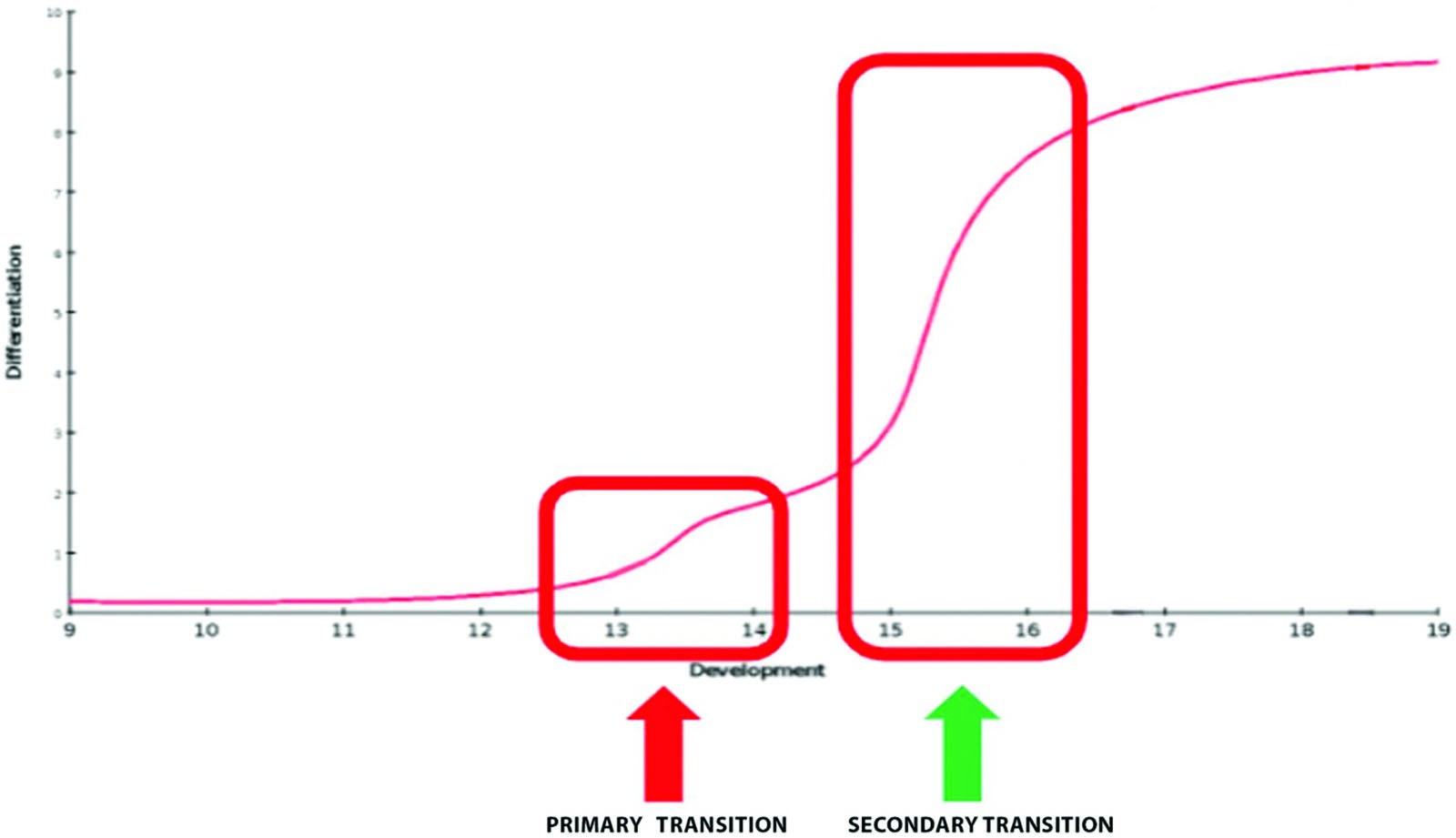
Based on the effects of GLP-1 in the adult, we hypothesize that mice without GLP-1 receptors would have decreased islet cell mass and reduced proliferation. As illustrated in Figure 2, different key developmental stages were studied in order to narrow down the role of GLP-1 during the early steps that control the endocrine fate, or the maturation of β-cells. If there is no difference between pups with GLP-1 receptor and without the GLP-1 receptor, we expect to see no difference in mass nor proliferation of cells.
Methods
Immunostaining
The project is centered on the analysis of the pancreatic phenotype of transgenic mice models. After newborn pancreatic dissection, the tissue is fixed, paraffin embedded and sectioned. For staining, the slides are deparaffinized and rehydrated before staining with different specific primary antibodies. The antibodies are chosen to target specific proteins in the pancreatic islets (e.g., Insulin, Glucagon, or Ki67 for proliferation quantification). A secondary antibody with a fluorophore allows us to visualize the staining in the islets under a fluorescent microscope. Primary antibodies are diluted in a blocking solution which prevents the antibodies from binding to any non-specific antigen. Slides are incubated with the antibody overnight at 4°C. After a series of washes, the secondary antibody (also diluted in blocking solution) is incubated for 2 hours at room temperature. After additional washes, the slides can be mounted with a glass coverslip using an anti-fading mounting solution and kept in the refrigerator until image acquisition. If scanning for proliferation, a DAPI counterstain is used when mounting the slides on glass. The DAPI counterstain allows us to stain the nuclei in blue.
Image Acquisition
Image acquisition is done with a fluorescence microscope with a motorized stage (Nikon AZ-100). Pictures are taken in three separate color channels (green, red, and blue) with a cooled monochrome camera with high bit depth. FITC secondary antibodies are used to stain green, Cy3 for red, and the DAPI counterstain for the blue channel. In our experiments, FITC was used for insulin in the β-cells in the pancreatic islets. Cy3 was used for either glucagon in the α-cells of the islets or for Ki67 (a proliferation marker) and DAPI provided a blue counterstain for the nuclei.
Morphometric Analysis
As previously described, after the images are captured on the microscope, they are further processed using Fiji, an image processing software used for morphometric analysis of the images (proliferation, β- and α-cell mass) (Cras-Méneur et al., 2016; Elghazi et al., 2017; Elghazi et al., 2017; Schindelin et al., 2012). To determine β-cell mass, Fiji is used to outline the boundaries of the pancreas and eliminate extraneous material in the photo. Next, the threshold feature is used to highlight the tissue and quantify the area of insulin positive staining of the pancreatic section. The β-cells are outlined using the insulin staining in the green channel and use thresholding to get the area of the β-cells. The same process is followed for α-cells in the red channel.
The total area of the α- or β-cells can be divided by the whole pancreatic area to calculate the percentage of the pancreas that consists of β- or α-cells. The proportion of staining and the whole pancreas weight allow for the determination of the mass of the corresponding cell type. Proliferation is measured using the Ki67 stain. Ki67 is used to measure proliferation because it is exclusively expressed during interphase of mitosis, and it binds to DNA. Ki67 is not present in G0 cells, but is expressed in G1, G2, S and M phase (Sun, X, & Kaufman, 2014). Ki67-expressing nuclei can be automatically counted in Fiji using different plugins allowing to reinforce contrast using Limited Adaptive Histogram Equalization (CLAHE) and identify the nuclei based on their sizes, roundness, staining intensity, and distance from one another using segmentation through an additional plugin (ITCN https://imagej.nih.gov/ij/plugins/itcn.html) (Zuiderveld, 1994). The percentage of proliferation was found in the pancreas through the ratio between proliferating cells expressing Ki67 in red and the total number of nuclei counterstained with DAPI in blue. This also allows examination of β-cell proliferation. This can be achieved by outlining the β-cells in all three channels based on the insulin staining and deleting the surrounding material. The blue channel and nuclei counter help determine the total number of nuclei in the β-cells and the red channel is used to determine which cells are proliferating within the β-cells.
Because the binomial distribution of the data could not be demonstrated, the data was analyzed using nonparametric tests (Mann Whitney) using the statistical analysis software Prism 9 (Graphpad Inc.). Prism was used to plot data and compare the differences between GLP-1 knockout mice and GLP-1 mice at different stages of the development and at birth.
Results
Reduced α- and β-cell mass in GLP-1R KO mice at birth
The data gathered for this experiment involved pancreatic sections 4 μm thick from five mice. Data was collected from 5 independent pancreatic sections. Sections were stained for insulin, glucagon, proliferation (Ki67), and nuclei (DAPI). Sections were isolated, stained, and analyzed for these factors to determine the role of GLP-1 during development. β- and α-cell mass is determined by isolating the cells and determining their mass relative to the entire section. The CLAHE tool is used to determine proliferation in the blue and red channels to count the number of proliferating nuclei.
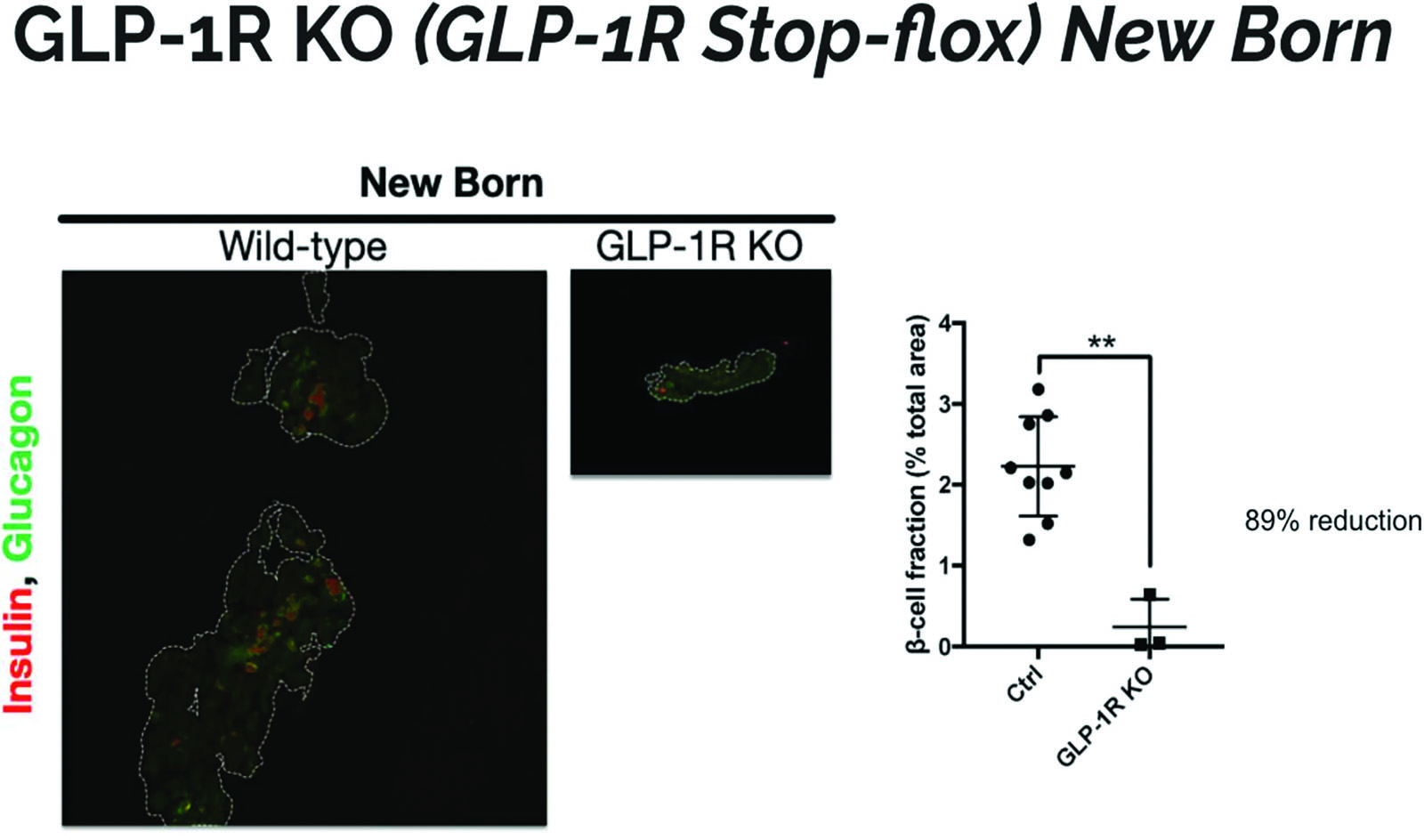
The pancreas sections for the GLP-1R KO mice on average were much smaller than sections for the wild mice. There was also more fluorescence in the wild type mouse and more cells stained positive for insulin and glucagon. Figure 3 which shows that there is an 89% reduction in β-cell fraction in GLP-1R KO mice. The difference in size could constitute for the higher expression of β−cells in the wild type mouse. Differentiated β-cells release insulin which we can measure through immunostaining. Since the GLP-1R KO mice have less β-cell mass, the results suggest that GLP-1 has a role in differentiation and growth of the pancreas.
Overall α-and β-cell proliferation is also reduced in KO mice
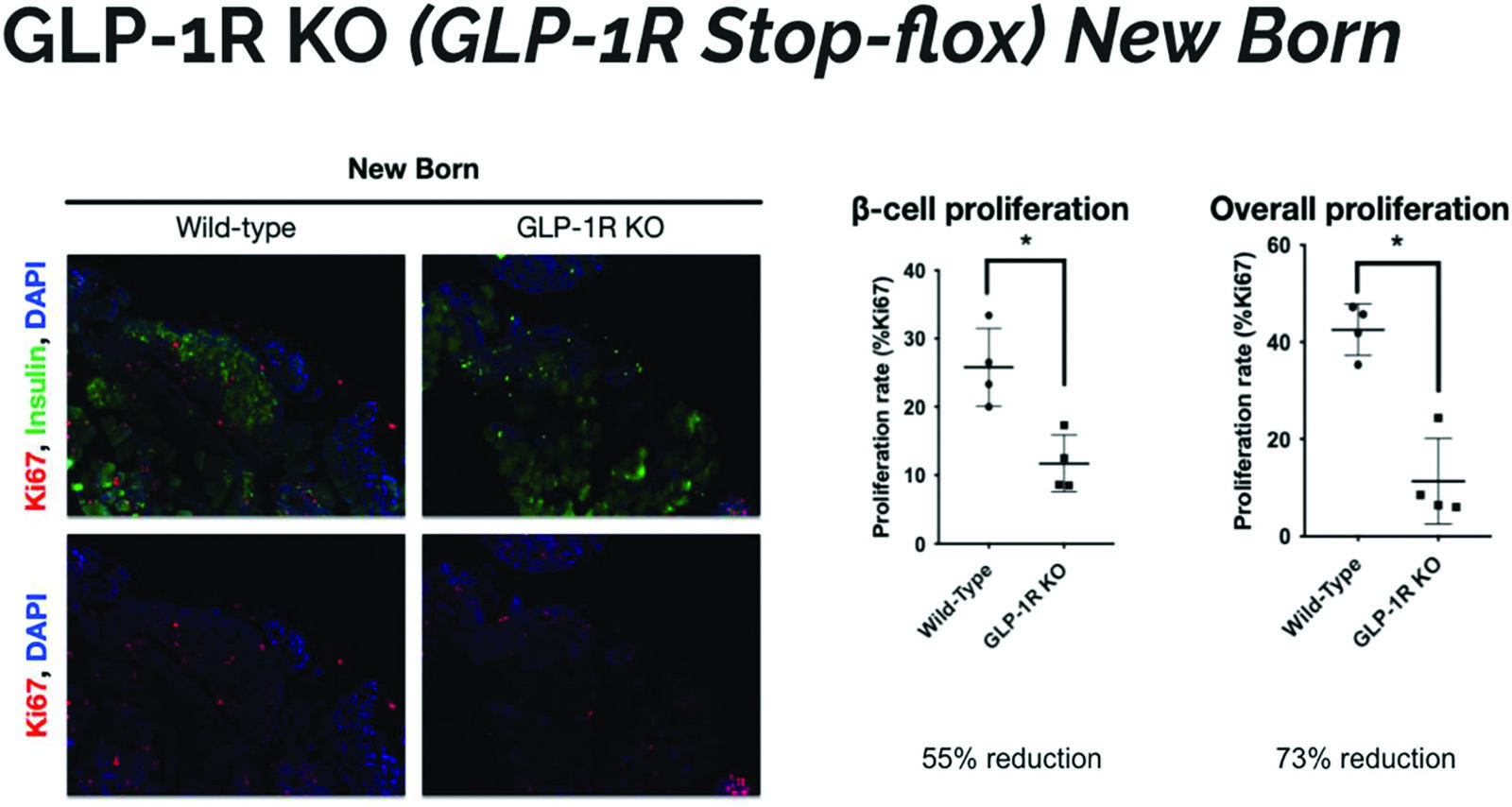
The wild type mice appear to have more red staining, indicative of Ki67, a marker of proliferation. The wild type mice have an increased percentage of positively stained cells which indicates that they have increased proliferation in islet cells (Figure 4). This indicates that the GLP-1R KO mice have less β−cell proliferation as well as less overall proliferation. This result shows that pancreatic cells in KO mice are not dividing as often and can account for the smaller size of the pancreas seen in Figure 1. GLP-1R KO mice are shown to have less pancreatic growth and islet cell concentration in comparison to their control counterparts.
The gathered data demonstrates that the GLP-1R KO mice exhibit less proliferation and reduced overall islet cell mass (both β and α) than their control counterparts at birth. The fewer number of cells could be due to reduced proliferation or increase apoptosis.
Discussion
These findings suggest that the GLP-1 protein has a role in the development of the pancreas. Considering the smaller size, decreased proliferation, and decreased islet mass in both α and β− cells, GLP-1 seems to be involved in development at birth. Concomitantly, both α- and β-positive cells were drastically reduced in the GLP-1R KO mice compared to the control, therefore increasing the deficit in islet mass at birth. The mass of the pancreas itself was less in the GLP-1R KO mice as well. The proliferation rate was also lower, suggesting that the GLP-1 protein has a role in pancreatic growth and differentiation during development, resulting in reduced mass and differentiation at birth. Further analysis will need to be performed to find out the exact timing GLP-1 is acting during development.
As a newborn, a glucose tolerance test is impractical, but due to decreased mass, we can anticipate that insulin and glucagon secretions might be impaired in these animals after birth. Further studies could block the GLP-1R on specific cells in the islets to determine if a certain type of cell in the pancreas has a larger role in development and differentiation. This research could investigate if GLP-1 has a role in paracrine signaling. The paracrine effect is the process by which one cell influences the differentiation of another nearby. Another potential direction for research could be investigating the effect of GLP-1 on cell differentiation. Islet cells can differentiate into either α-, β-, δ-, ε-, and PP cells. Differentiation is a key part of development, and this research could further uncover the function of the GLP-1 protein.
It is hypothesized that GLP-1 assists in the differentiation of pancreatic cells during organogenesis. Further studies at key embryonic stages (see Figure 2) could determine whether GLP-1 could affect pancreatic mass by altering the proliferation or the differentiation of the progenitors. This hypothesis could be studied by culturing pancreatic progenitor cells in the presence and absence of GLP-1. Cells can be marked for proliferation with Ki67. If proliferation of pancreatic progenitors is equal across conditions, then GLP-1 does not play a role in proliferation of progenitors. It is also possible that GLP-1 absence could be decreasing the endocrine mass of the pancreas by reducing differentiation of islet cells. In a similar setup, progenitors can be cultured with and without GLP-1. Cells can be stained for determinants of differentiation, such as staining for insulin in β cells. Studies of this type are limited because of the α-cells ability to secrete GLP-1. To account for this, future studies could genetically modify mice and delete the genes coding for secretion of GLP-1. The results of this study can give researchers important information on pancreatic development with GLP-1 and can help make strides towards the therapeutic treatment of diabetes that could further be adapted for the in vitro differentiation of embryonic stem cells towards β-cells (Millman et al., 2016; Pagliuca et al., 2014).
References
Alejandro, E. U., Gregg, B., Wallen, T., Kumusoglu, D., Meister, D., Chen, A., Merrins, M. J., Satin, L. S., Liu, M., Arvan, P., & Bernal-Mizrachi, E. (2014). Maternal diet-induced microRNAs and mTOR underlie beta cell dysfunction in offspring. J Clin Invest, 124(10), 4395–4410.
Barrera, J. G., D’Alessio, D. A., Drucker, D. J., Woods, S. C., & Seeley, R. J. (2009). Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes, 58(12), 2820–2827.
Cras-Méneur, C., Conlon, M., Zhang, Y., Pasca di Magliano, M., & Bernal-Mizrachi, E. (2016). Early pancreatic islet fate and maturation is controlled through RBP-Jκ. Scientific reports, 6, 26874.
Cras-Méneur, C., Elghazi, L., Czernichow, P., & Scharfmann, R. (2001). Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: a balance between proliferation and differentiation. [In Vitro]. Diabetes, 50(7), 1571–1579.
Cras-Méneur, C., & Scharfmann, R. (2002). FGFR1-IIIb is a putative marker of pancreatic progenitor cells. Mechanisms of development, 116(1–2), 205–208.
D’Alessio, D. A., Verchere, C. B., Kahn, S. E., Hoagland, V., Baskin, D. G., Palmiter, R. D., & Ensinck, J. W. (1994). Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes, 43(12), 1457–1461.
Drucker, D. J., & Nauck, M. A. (2006). The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet, 368(9548), 1696- 1705.
Elghazi, L., Blandino-Rosano, M., Alejandro, E., Cras-Meneur, C., & Bernal-Mizrachi, E. (2017). Role of nutrients and mTOR signaling in the regulation of pancreatic progenitors development. Mol Metab, 6(6), 560–573.
Elghazi, L., Blandino-Rosano, M., Alejandro, E., Cras-Méneur, C., & Bernal-Mizrachi, E. (2017). Role of nutrients and mTOR signaling in the regulation of pancreatic progenitors development. Molecular Metabolism, 0(0), 560–573.
Elghazi, L., Cras-Méneur, C., Czernichow, P., & Scharfmann, R. (2002). Role for FGFR2IIIb- mediated signals in controlling pancreatic endocrine progenitor cell proliferation. Proceedings of the National Academy of Sciences of the United States of America, 99(6), 3884–3889.
Gromada, J., Ding, W. G., Barg, S., Renstrom, E., & Rorsman, P. (1997). Multisite regulation of insulin secretion by cAMP-increasing agonists: evidence that glucagon-like peptide 1 and glucagon act via distinct receptors. Pflugers Arch, 434(5), 515–524.
Light, P. E., Manning Fox, J. E., Riedel, M. J., & Wheeler, M. B. (2002). Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol, 16(9), 2135–2144.
Millman, J. R., Xie, C., Van Dervort, A., Gürtler, M., Pagliuca, F. W., & Melton, D. A. (2016). Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nature Communications, 7, 11463.
Pagliuca, F. W., Millman, J. R., Gürtler, M., Segel, M., Van Dervort, A., Ryu, J. H., Peterson, Q. P., Greiner, D., & Melton, D. A. (2014). Generation of Functional Human Pancreatic β Cells In Vitro. Cell, 159(2), 428–439.
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., & Cardona, A. (2012). Fiji: an open-source platform for biological- image analysis. Nature Methods, 9(7), 676–682.
Smith, E. P., An, Z., Wagner, C., Lewis, A. G., Cohen, E. B., Li, B., Mahbod, P., Sandoval, D., Perez-Tilve, D., Tamarina, N., Philipson, L. H., Stoffers, D. A., Seeley, R. J., & D’Alessio, D. A. (2014). The role of beta cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab, 19(6), 1050–1057.
Sun, X., & Kaufman, P. D. (2018). Ki-67: more than a proliferation marker. Chromosoma, 127(2), 175–186.
Talchai, S. C., & Accili, D. (2015). Legacy Effect Of Foxo1 In Pancreatic Endocrine Progenitors On Adult β-cell Mass And Function. Diabetes, 64(8), 2868–2879.
Thorens, B., Dériaz, N., Bosco, D., DeVos, A., Pipeleers, D., Schuit, F., Meda, P., & Porret, A. (1996). Protein kinase A-dependent phosphorylation of GLUT2 in pancreatic beta cells. The Journal of biological chemistry, 271(14), 8075–8081.
Villanueva-Peñacarrillo, M. L., Puente, J., Redondo, A., Clemente, F., & Valverde, I. (2001). Effect of GLP-1 treatment on GLUT2 and GLUT4 expression in type 1 and type 2 rat diabetic models. Endocrine, 15(2), 241–248.
Whalley, N. M., Pritchard, L. E., Smith, D. M., & White, A. (2011). Processing of proglucagon to GLP-1 in pancreatic alpha-cells: is this a paracrine mechanism enabling GLP-1 to act on beta-cells? J Endocrinol, 211(1), 99–106.
Zuiderveld, K. (1994). Contrast limited adaptive histogram equalization. In I. Academic Press Professional (Ed.), Graphics gems IV (pp. 474–485).