Introduction
Typhoid fever, a result of the Salmonella enterica serovar Typhi (S. typhi), is a bacterial infection that largely spreads through contamination in food and water, as well as by close contact. Those infected by S. typhi may develop typhoid fever (typhoid), displaying many cold-like symptoms in addition to more severe gastrointestinal, muscle, and life-threatening states. Fevers of up to 103–104°F (39–40°C) are commonplace alongside coughs, stomachaches, and lethargy.1,2 South Asia is a leading region in the world in the number of typhoid cases, with a significant portion of populations at risk, including immunocompromised adults and children. Pakistan has the highest incidence of typhoid among its neighboring countries, averaging 493.5 cases per 100,000 in 2018.3 The country suffers from issues of overcrowding, inadequate sanitation and healthcare, and insufficient access to clean water, ranking at 35.84% availability of clean water in 2020.4 Deteriorating living conditions enable food and water sources to become contaminated with feces that contain bacteria, with poor water, sanitation, and hygiene infrastructure contributing to the spread of the illness.
The virulence and spread of S. typhi have been influenced by its ability to endure for long periods of time outside of a human host, such as in water or on surfaces, as well as other genetic factors. In 2016, the emergence of a new extensive drug-resistant (XDR) strain of typhoid in Hyderabad known as H58 resulted in the need for stronger antimicrobials to combat the pathogen. The advent of COVID-19 exacerbated the public health crisis because overlapping symptoms made identification of case etiology difficult, as well as explorations of treatment options for COVID-19, including azithromycin, which could inadvertently increase resistance to typhoid across the scope.5
In this article, we conduct a comprehensive review of the literature on the state of XDR-typhoid in the post-COVID-19 era, develop insights on the conditions that promoted the spread of the disease, and highlight recommendations to reduce the burden of the disease. We look through databases, including PubMed and Google Scholar, to find relevant research articles, academic journals, and authoritative reports. This was filtered down to those pertinent to the study’s geographical reason, with the review serving as the foundation of knowledge regarding the state of XDR-typhoid and its implications for global health. In addition, data collection was a crucial part of the study, with reliable and up-to-date information on XDR-typhoid cases, epidemiological trends, and public health interventions gathered from reputable sources such as government health agencies, international organizations, and research institutions. The government of Pakistan’s own reporting of the epidemic and national trends through its weekly field epidemiological reports was particularly useful. The collected data was then analyzed and synthesized to identify key trends, patterns, and insights, shedding light on the factors that contributed to the spread of XDR-typhoid and the challenges faced in managing the disease. Furthermore, the research aimed to identify any knowledge gaps or areas that required further investigation, guiding future research endeavors.
Socioeconomic Context and Water, Sanitation, and Hygiene in Pakistan
Issues ranging from contaminated water being used in drinking and irrigation sources, as well as the overall weak socioeconomic status of many Pakistanis, exacerbate the spread of typhoid.9 In Pakistan, economic turmoil and foreign debts, which leave just $151 million for the country’s whole healthcare system, are important contributors to the outbreak of typhoid. Given that over 40% of the population that falls below the poverty line reside in slums and congested regions, the current rates of investment in healthcare fall acutely short of fulfilling the country’s needs.10 Airborne infections are caused by inadequate sanitation facilities, lack of clean food supply, and inaccessibility of clean drinking water.11 In addition, insufficient money prevents the provision of acceptable residential infrastructure and common sanitary practices despite the development of basic health services established by government agencies in neighboring municipalities. Thus, a lot of doctors hesitate to work in rural regions. As a result, many people are unable to access medical care or guidance, increasing the incidence and mortality of typhoid.12
Potential carriers from impoverished regions go to urban centers in pursuit of secure employment or as a last resort to escape poverty, leading to an increase in typhoid fever cases among city residents.11 Although plenty of individuals prefer food from the streets, there are significant rates of typhoid infections that are brought on as a result. Estimates range from 70% to 90% in reports of seeing animals, flies, other insects, or liquid waste in locations where meals are prepared.13 Food goods are frequently contaminated by dust and animal droppings when carts are parked near or over drains. Drains can be a source of water and food remnants, thus attracting insects, rodents, birds, as well as pests and other contaminants if not cleaned properly. There is also the potential for airborne transmission through windblown particles of dust and debris entering food products. Many vendors handle food without following standard sanitary procedures, such as cleaning their hands with soap and water after handling raw ingredients, cash, or food packaging.14 Cross-contamination is common since improperly cleaned surfaces can still have food preparation residue on them.
Most rural communities do not have access to services like faucets, wash basins, and individual hygiene supplies; thus, rivers and water wells are the main sources of drinking water there. In addition, a lot of the water they receive is not fit for human consumption because it has not undergone quality testing and may have high bacterial loads.15 Furthermore, companies that use their property as a landfill pollute their water supplies. All of these elements work together to severely pollute the environment and contaminate their sole water supplies, raising the possibility of developing Salmonella infections. Due to poor personal as well as sanitary hygiene practices, rural communities are more susceptible to typhoid. This is clearly given in that over 25 million individuals in Pakistan practice open defecation in vegetation, waterways, and on highways, further increasing the potential for pathogen transmission.16
Karachi has been noted to also have the presence of many microbial contaminants within its various water supplies, suggesting how the consumption of water by the 16 million residents or so of the city can be a means of advancing the spread of the disease.20 The unhealthy effects of it are notable, and the increase in population in the city center as a result of migration from rural outskirts to urban areas has led to predictions of the population projecting to be 23.1 million strong by the year 2035.21 A significant section of the country’s population is now forced to reside in impoverished and congested places, where access to necessities like a functioning sewage system, drinkable water, and clean food is simply out of reach. To make matters worse, residents in these places are less informed about fundamental hygiene principles and the transmission of infectious illnesses, which leaves them more open to the dangers of typhoid fever. In addition, these communities’ residents lack simple access to institutions that provide economical and high-quality healthcare. Furthermore, the existing antibiotic resistance is getting worse due to a shortage of qualified medical staff working in such institutions.
Spread of Typhoid in Pakistan
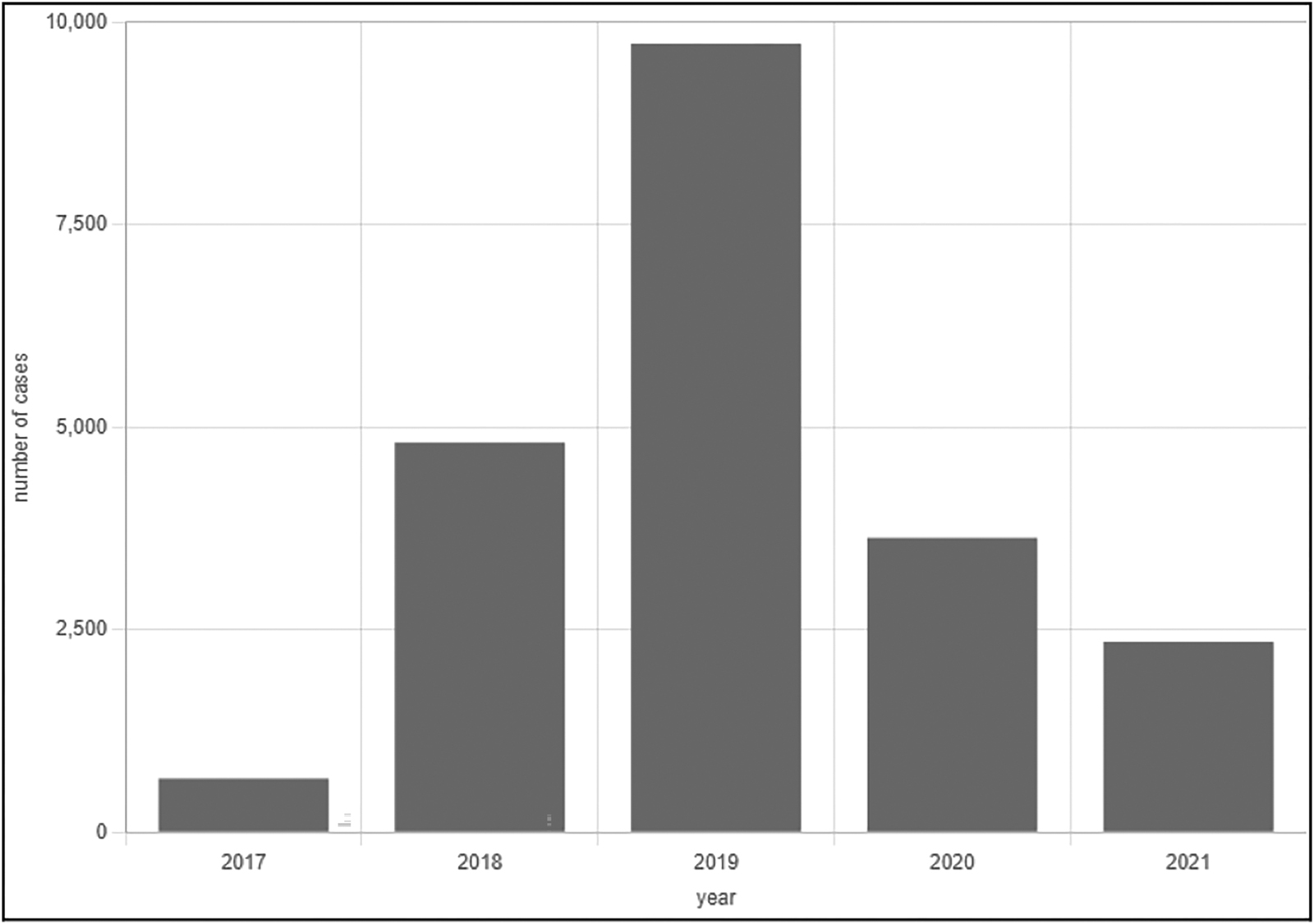
The end of 2016 saw the advent of the first recorded case of an XDR strain of typhoid as it emerged from Sindh, Pakistan. Initial reports placed it in conjunction with the massive influx of typhoid fever cases that had been confirmed via blood culture and acted refractory to standard therapy.6 From 2016 to 2021, the National Institute of Health (NIH) Islamabad Weekly Field Epidemiological Report reported a total of 14,360 XDR-typhoid cases in Karachi alone, the largest city and capital of the Sindh province. During a similar period spanning from November 2016 to June 2021, a total of 5,741 confirmed XDR-typhoid cases were reported in all the remaining districts of Sindh, discounting Karachi. Of those, 69.5% of cases came from the Hyderabad District.7 As a result of this, Pakistan began spearheading the implementation of the typhoid conjugate vaccine as a part of its routine childhood immunization program, as per the guidelines stipulated by the World Health Organization (WHO).6
Fear of international outbreaks and spillovers from the currently multidrug-resistant strain of typhoid fever in Pakistan remains at the forefront of the concern of many global actors.
The onset of the COVID-19 pandemic drastically altered the landscape of the discourse around typhoid fever as it began to pose possibilities of co-epidemics and co-infections.17,18 The healthcare infrastructure in Pakistan has been noted to be already burdened beyond its capacity, and several pulmonological conditions including acute respiratory distress syndrome, pneumothorax, or even the advent of other cardiovascular diseases and secondary infections can drive the fragile system into collapse.19 Such scenarios came to a head when 20,000 typhoid cases were reported to have occurred within a span of 10 days in the summer of 2020 with co-infections of SARS-CoV-2.5
Healthcare practitioners all around the country are finding it difficult to control the XDR-typhoid strain given the variety of challenges faced. Azithromycin, an antibiotic used to treat a wide range of bacterial infections, is still the sole accessible oral treatment choice among the three medicines frequently used to treat XDR-typhoid fever, making it the final line of defense for treating patients in an outpatient environment. Nevertheless, XDR-typhoid is becoming more resistant to the administration of azithromycin, according to recent investigations.22 As a result of this, physicians are being forced to inject both tigecycline and carbapenem, which are typically reserved as the last-resort treatment for typhoid fever. Due to their high cost and relative inaccessibility to the impoverished population in developing nations, many of these medications require in-patient treatment.11 In addition, when instances of XDR-typhoid fever epidemics started to climb at an alarming rate, authorities in a number of nations expressed worry over the coming global impact of these outbreaks. Travel restrictions and guidelines are already becoming commonplace as seen by CDC recommendations urging all travelers to this region of the world to have a typhoid vaccination as a precaution.23
Multi-drug-resistant Strain of S. typhimurium—Haplotype 58
Typhoid fever is primarily caused by the bacterium, Salmonella enterica serovar Typhi, which is able to spread easily due to a variety of factors. The presence of the typhoid toxin that can bind to a multitude of cells and its possession of a polysaccharide capsule aids its ability to rebuff the immune system and survive in harsh environments, such as an acidic stomach. S. typhi demonstrates the capacity to generate virulence factors, encompassing toxins and enzymes, facilitating its intracellular invasion and replication within human cells.
There are a variety of strains of Salmonella enterica serovar Typhi, all of which have varying degrees of resistance to antibiotics. Initially, most S. typhi strains were susceptible to first-line antimicrobial therapy; however, in 2011, there was a drastic increase in a new group of multidrug-resistant strains of S. typhi, known as Haplotype 58, or H58.24
H58 is a specific genetic variant of S. typhi, which arose through genetic mutations.
Unlike other strains of S. typhi, H58 strains display more resistance to current treatment methods, such as Fluoroquinolones, ampicillin, and trimethoprim-sulfamethoxazole, which have often been used to treat typhoid fever. They also display a wide range of genetic diversity, as well as increased fitness and transmission, which has allowed strains of H58 to be associated with several large-scale outbreaks in recent years. The typhoid outbreak within Pakistan can be attributed to Haplotype 58, and despite public health methods being in play, the drug resistance and fitness of strains of H58 have allowed it to rapidly spread and lead to such outbreaks.
Molecular Structure and Drug Resistance of S. typhi Haplotype 58
Analyzing the molecular and genetic structure of typhoid is essential to understanding its effectiveness in spreading so quickly within Pakistan. As mentioned, the salmonella bacterium is transmitted directly through food and water. Salmonella is characterized as a flagellated facultatively anaerobic bacilli.25 Salmonella is able to reach the intestinal epithelium, surviving through the gastrointestinal tract. It can survive the acidic environment of the stomach, largely in part to its ability to produce acid shock proteins and its strong outer membrane and cell wall. The outer membrane of Salmonella is asymmetric, with the inner layer consisting of a phospholipid layer and the outer layer a lipopolysaccharide layer, which is divided into three regions: lipid A, core, and O polysaccharides.26 This complex outer region of Salmonella has been suggested to have provided high virulence since the polysaccharide layer has a number of benefits, including but not limited to retaining water, providing protection against antibiotics, and containing efflux pumps that can remove antibiotics Jamilah. This gives Salmonella a high level of survivability, which has been essential in allowing it to spread throughout Pakistan.
Haplotype 58 has the above structural characteristics, but there are a number of genetic mutations that have provided it with even more increased resistance. These mutations consist of genes that code for polysaccharides and a more effective typhoid toxin, which causes typhoid fever.26 Furthermore, Haplotype 58 strains have undergone a mutation that causes increased expression in the rpoS gene, which is a stress response regulator. This allows increased survivability in extreme environments and more resistance to antibiotics due to the release of pertinent proteins for protection.27 To conclude, Haplotype 58 has expressed a variety of mutations, many not yet understood, that have directly led to increased transmission and drug resistance, which has made it difficult to contain its spread within Pakistan and provide potential treatments to the people.
Conclusions
Treatment, Preventative Measures, and Recommendations
Vaccination is at the forefront of efforts attempting to combat XDR-typhoid fever cases in Pakistan. Vaccines can be administered to children who are 6 months old with long-lasting effects, which is quite relevant, given that 60–70% of typhoid-related cases and deaths in Pakistan were in children under the age of 15, per a 2017 study.28 The efficacy of the vaccines is also undoubtedly evident, as the number of cases of typhoid fever in unvaccinated children was over double that in vaccinated children.12 Furthermore, according to the information obtained from Pakistan, the typhoid fever vaccine is 95% effective directed at Salmonella typhi strains that have been verified by culture, and it is 97% effective versus strains that have become resistant to the vaccine.29 Such efficacy rates underline the vaccine’s potency and may contribute to a decrease in the overuse of antibiotics that caused the establishment of several different strains of typhoid. XDR was initially noted to be within the southern province of Sindh, where a two-week vaccination program had been implemented in 2019 with the goal of immunizing 10 million children between the ages of 9 months and 15 years old. Two years later in Punjab, the subsequent stage of the vaccination drive was started with the goal of immunizing upward of 6.6 million kids who fell within a similar age range.30 The success of these efforts being championed by the WHO in tandem with the Pakistani government is undoubted, as multidrug-resistant typhoid cases have decreased by two-thirds-fold in Sindh and rates of vaccination in Punjab accelerated (World Health Organization, 2021).31
With this critical information in mind, a couple of recommendations can be sorted (see Table 1).
Recommendations for XDR-Typhoid Prevention and Management10
Recommendations |
Individuals |
Local Government |
NGOs |
|---|---|---|---|
Basic hygiene practices |
X |
X |
X |
Implementing health classes on typhoid fever in schools |
X |
X |
|
Collaboration with state governments and local hospitals to spread awareness |
X |
X |
|
Public postings on the effects of typhoid fever and preventative measures |
X |
X |
|
The National Assembly should pass bills that raise the threshold for physicians employing prescriptions |
X |
||
Azithromycin should be reserved for a case-by-case basis |
X |
||
Allocating bonuses for healthcare workers in rural areas to incentivize working in these communities |
X |
||
Investing in an integrated accessible telehealth system to allow reduction in costs of transport from remote areas |
X |
||
Increasing the supply of diagnostic tools and testing kits |
X |
X |
|
Passing stronger laws on the prohibition of open defecation |
X |
||
Government-subsidized workshops on constructing water filters, with priority in rural areas |
X |
||
Long-term investment in the education sector to ensure meals and other resources are provided to children |
X |
X |
|
Construct sanitation centers in publicly accessible areas of Pakistan that are regularly maintained |
X |
X |
|
Distribution of chlorine tablets to slums and neighborhoods to ensure soap and cleaning products are available |
X |
X |
|
Developing an integrated system of sewage disposal that enables rainwater to be stored and harvester for local drinking |
X |
X |
|
Financing efforts for healthcare workers to go to different neighborhoods to provide an in person training on health practices |
X |
The scope of these recommendations varies, highlighting the collaborative efforts required to tackle the public health crisis effectively. Basic hygiene practices are emphasized at the individual level, promoting personal responsibility in preventing transmission. At the local government level, collaboration with state governments and hospitals is suggested to disseminate awareness about typhoid and its preventive measures, indicating a more community-oriented approach. NGOs play a vital role in multiple areas, including distributing hygiene information and supplies, supporting healthcare workers in rural areas, and investing in telehealth systems for remote access to healthcare services. Moreover, local governments and NGOs share responsibilities in initiatives like constructing sanitation centers and distributing chlorine tablets, targeting improved sanitation and hygiene facilities in both urban and rural areas. These recommendations collectively underscore the need for a multifaceted approach, with contributions from various entities, to combat XDR-typhoid effectively and are the first steps toward a longer strategy aimed to combat and reduce the burden of disease spread found in Pakistan.12 Mass vaccination campaigns continue to be expensive, placed at $1.50 per dose, and need a stronger government and healthcare force at the federal and provincial levels for successful implementation. Social media posts also have many restrictive ends to them, given many of the impoverished (who are more susceptible to typhoid fever) do not have access to technology and applications in the way that others of a higher socioeconomic class are. Non-governmental organizations such as Coalition Against Typhoid (CaT), marked in financial transparency, continue to operate and promote awareness of typhoid fever’s spread, yet these efforts do not meet the minimum threshold to significantly reduce Pakistan’s prevailing public health crisis. A robust and forward-thinking approach needs to be taken by the Pakistani government to use its budget and international funding in the sectors of health and education to ensure disease prevention. It is crucial to understand that XDR-typhoid is a symptom of larger issues with global health security rather than a stand-alone issue. Antibiotic-resistant illnesses are an increasing global health challenge; thus, efforts to control XDR-typhoid must be viewed as a component of a bigger plan to address this issue. This entails funding the discovery and advancement of novel therapies, encouraging prudent antibiotic usage, and bolstering health systems to enhance the provision of care for infectious illnesses. In addition, it is imperative to make certain that the effort to respond to XDR-typhoid is equitable and just, considering the requirements of populations who are vulnerable and those who live in places with a lack of resources. This necessitates a focus on social justice and health equity, as well as an understanding that some communities bear a disproportionate amount of the burden of sickness. The core causes of health disparities, such as poverty and prejudice, which contribute to the development of contagious illnesses like XDR-typhoid, must also be addressed.
References
1. Bhandari, J., Thada, P.K., DeVos, E. Typhoid Fever. [Updated 2022 Aug 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 January. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557513/https://www.ncbi.nlm.nih.gov/books/NBK557513/
2. Centers for Disease Control and Prevention. (2022, December 12). Symptoms and treatment. Centers for Disease Control and Prevention. Retrieved April 24, 2023, from https://www.cdc.gov/typhoid-fever/symptoms.htmlhttps://www.cdc.gov/typhoid-fever/symptoms.html
3. Fatima, M., Kumar, S., Hussain, M., Memon, N. M., Vighio, A., Syed, M. A., … & Khader, Y. (2021). Morbidity and mortality associated with typhoid fever among hospitalized patients in Hyderabad district, Pakistan, 2017–2018: retrospective record review. JMIR Public Health and Surveillance, 7(5), e27268.
4. Pakistan Clean Water Access 2000–2023. MacroTrends. (n.d.). Retrieved April 24, 2023, from https://www.macrotrends.net/countries/PAK/pakistan/clean-water-access-statisticshttps://www.macrotrends.net/countries/PAK/pakistan/clean-water-access-statistics
5. Ahmad, S., Tsagkaris, C., Aborode, A. T., Ul Haque, M. T., Khan, S. I., Khawaja, U. A., Carla Dos Santos Costa, A., Essar, M. Y., & Lucero-Prisno, D. E., 3rd (2021). A skeleton in the closet: The implications of COVID-19 on XDR strain of typhoid in Pakistan. Public Health in Practice (Oxford, England), 2, 100084. https://doi.org/10.1016/j.puhip.2021.100084https://doi.org/10.1016/j.puhip.2021.100084
6. Akram, J., Khan, A. S., Khan, H. A., Gilani, S. A., Akram, S. J., Ahmad, F. J., & Mehboob, R. (2020). Extensively drug-resistant (XDR) typhoid: evolution, prevention, and its management. BioMed Research International, 2020.
7. Federal Disease Surveillance and Response Unit Field Epidemiology & Disease Surveillance Division. (2021, June 23). Weekly Field Epidemiology Report – National Institute of Health, Islamabad. Retrieved April 24, 2023, from https://www.nih.org.pk/wp-content/uploads/2021/06/25-FELTP-Pakistan-Weekly-Epidemiological-Report-June-13-19-2021-.pdfhttps://www.nih.org.pk/wp-content/uploads/2021/06/25-FELTP-Pakistan-Weekly-Epidemiological-Report-June-13-19-2021-.pdf
8. Federal Disease Surveillance and Response Unit Field Epidemiology & Disease Surveillance Division. (2021, August 25). Weekly Field Epidemiology Report – National Institute of Health, Islamabad. Retrieved April 24, 2023, from https://www.nih.org.pk/wp-content/uploads/2021/08/34-FELTP-Pakistan-Weekly-Epidemiological-Report-Aug-15-21-2021-.pdfhttps://www.nih.org.pk/wp-content/uploads/2021/08/34-FELTP-Pakistan-Weekly-Epidemiological-Report-Aug-15-21-2021-.pdf
9. Hafeez, S., Din, M., Zia, F., Ali, M., & Shinwari, Z. K. (2021). Emerging concerns regarding COVID‐19; second wave and new variant. Journal of Medical Virology, 93(7), 4108.
10. Tienne. (2022, April 25). Typhoid fever in Pakistan. ArcGIS StoryMaps. Retrieved April 24, 2023, from https://storymaps.arcgis.com/stories/461001cedbd44c7aa67dead005364090https://storymaps.arcgis.com/stories/461001cedbd44c7aa67dead005364090
11. Butt, M. H., Saleem, A., Javed, S. O., Ullah, I., Rehman, M. U., Islam, N., … & Misbah, S. (2022). Rising XDR-typhoid fever cases in Pakistan: are we heading back to the pre-antibiotic era?. Frontiers in Public Health, 9, 794868.
12. Tharwani, Z. H., Kumar, P., Salman, Y., Islam, Z., Ahmad, S., & Essar, M. Y. (2022). Typhoid in Pakistan: challenges, efforts, and recommendations. Infection and Drug Resistance, 15, 2523–2527. https://doi.org/10.2147/IDR.S365220https://doi.org/10.2147/IDR.S365220
13. Rane, S. (2011). Street vended food in developing world: hazard analyses. Indian Journal of Microbiology, 51(1), 100–106.
14. Raza, J., Asmat, T. M., Mustafa, M. Z., Ishtiaq, H., Mumtaz, K., Jalees, M. M., … & ur Rehman, H. (2021). Contamination of ready-to-eat street food in Pakistan with Salmonella spp.: implications for consumers and food safety. International Journal of Infectious Diseases, 106, 123–127.
15. Farooqui, A., Khan, A., & Kazmi, S. U. (2009). Investigation of a community outbreak of typhoid fever associated with drinking water. BMC Public Health, 9, 1–6.
16. Wash: Water, sanitation and hygiene. UNICEF Pakistan. (n.d.). Retrieved April 24, 2023, from https://www.unicef.org/pakistan/wash-water-sanitation-and-hygiene-0https://www.unicef.org/pakistan/wash-water-sanitation-and-hygiene-0
17. Shaikh, B. T., & Ali, N. (2020). COVID‐19 and fiscal space for health system in Pakistan: it is time for a policy decision. The International Journal of Health Planning and Management, 35(4), 813–817.
18. Haqqi, A., Khurram, M., Din, M. S. U., Aftab, M. N., Ali, M., Ahmed, H., & Afzal, M. S. (2021). COVID‐19 and Salmonella Typhi co‐epidemics in Pakistan: a real problem. Journal of Medical Virology, 93(1), 184.
19. Misbah, S., Ahmad, A., Butt, M. H., Khan, Y. H., Alotaibi, N. H., & Mallhi, T. H. (2020). A systematic analysis of studies on corona virus disease 19 (COVID-19) from viral emergence to treatment. J Coll Physicians Surg Pak, 30(6), 9–18.
20. Daud, M. K., Nafees, M., Ali, S., Rizwan, M., Bajwa, R. A., & Shakoor, M. B. & Malook, I. (2017). Drinking water quality status and contamination in Pakistan. BioMed Research International, 1–18.
21. National Intelligence Council. (2021, March). Global Trends 2040: A More Contested World. DNI.gov. Retrieved April 24, 2023, from https://www.dni.gov/files/ODNI/documents/assessments/GlobalTrends_2040.pdfDNI.govhttps://www.dni.gov/files/ODNI/documents/assessments/GlobalTrends_2040.pdf
22. Mansoor, H., Ahmed, K., Fida, S., Uzair, M., Asghar, A., Iqbal, J. (2020). Gastrointestinal and hepatobiliary complications of extensively drug-resistant typhoid at a Tertiary Care Hospital in Pakistan. Cureus, 12, e11055. doi: 10.7759/cureus.1105510.7759/cureus.11055
23. Centers for Disease Control and Prevention. (2021, September 30). Extensively drug-resistant typhoid fever in Pakistan - watch - level 1, practice usual precautions - travel health notices. Centers for Disease Control and Prevention. Retrieved April 24, 2023, from https://wwwnc.cdc.gov/travel/notices/watch/xdr-typhoid-fever-pakistanhttps://wwwnc.cdc.gov/travel/notices/watch/xdr-typhoid-fever-pakistan
24. Feasey, Nicholas A. et al. Rapid emergence of multidrug-resistant, H58-lineage Salmonella typhi in Blantyre, Malawi. PLoS Neglected Tropical Diseases, 9,4 e0003748. 24 Apr. 2015, doi:10.1371/journal.pntd.000374810.1371/journal.pntd.0003748
25. Jamilah, J., Hatta, M., Natzir, R., Umar, F., Sjahril, R., Agus, R., Junita, A. R., Dwiyanti, R., Primaguna, M. R., & Sabir, M. (2020). Analysis of existence of multidrug-resistant H58 gene in Salmonella enterica serovar Typhi isolated from typhoid fever patients in Makassar, Indonesia. New Microbes and New Infections, 38, 100793. https://doi.org/10.1016/j.nmni.2020.100793https://doi.org/10.1016/j.nmni.2020.100793
26. Giannella, R. A. Salmonella. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 21. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8435/https://www.ncbi.nlm.nih.gov/books/NBK8435/
27. V T Nair, D., Venkitanarayanan, K., & Kollanoor Johny, A. (2018). Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods (Basel, Switzerland), 7(10), 167. https://doi.org/10.3390/foods7100167https://doi.org/10.3390/foods7100167
28. Pakistan becomes first country to introduce new typhoid vaccine into routine immunisation program. Gavi, the Vaccine Alliance. (2019, November 15). Retrieved April 24, 2023, from https://www.gavi.org/news/media-room/pakistan-becomes-first-country-introduce-new-typhoid-vaccine-routine-immunisationhttps://www.gavi.org/news/media-room/pakistan-becomes-first-country-introduce-new-typhoid-vaccine-routine-immunisation
29. Yousafzai, M. T., Karim, S., Qureshi, S., Kazi, M., Memon, H., Junejo, A., … & Qamar, F. N. (2021). Effectiveness of typhoid conjugate vaccine against culture-confirmed Salmonella enterica serotype Typhi in an extensively drug-resistant outbreak setting of Hyderabad, Pakistan: a cohort study. The Lancet Global Health, 9(8), e1154–e1162.
30. OCHA. (2021, June 28). Who conducts typhoid vaccination campaign – Pakistan. ReliefWeb. Retrieved April 24, 2023, from https://reliefweb.int/report/pakistan/who-conducts-typhoid-vaccination-campaignhttps://reliefweb.int/report/pakistan/who-conducts-typhoid-vaccination-campaign
31. World Health Organization. (2021, March 10). Over 13 million children vaccinated with typhoid conjugate vaccine in Punjab and Islamabad. World Health Organization. Retrieved April 24, 2023, from https://www.emro.who.int/pak/pakistan-infocus/over-13-million-children-vaccinated-with-typhoid-conjugate-vaccine-in-punjab-and-islamabad.htmlhttps://www.emro.who.int/pak/pakistan-infocus/over-13-million-children-vaccinated-with-typhoid-conjugate-vaccine-in-punjab-and-islamabad.html