Introduction
The demand for efficient, renewable, and modern-day energy is more important than ever. Individually, energy is a fundamental part of today’s society, empowering the majority of our infrastructure. Currently, the majority of energy is produced by burning fossil fuels, comprising 80% of the world’s energy consumption in 2019 (Total energy consumption, 2021). And coupled with the declining quantity of fossil fuels, readily available methods of creating efficient, renewable energy are becoming a more important concern to be addressed. One potential technology that can aid in solving this issue is the Hydrogen Fuel Cell (HFC), a device that converts hydrogen fuel’s chemical energy into electrical energy. (Brandon and Thompsett, 2005: 59). Specifically, the Proton-Exchange Membrane Fuel Cell (PEMFC), which is more compact than other HFCs, has the potential to be implemented globally as a clean power source. Before its implementation, the PEMFC needs to have maximal efficiency to ensure easy integration into our daily use.
This investigation analyzes the methods in which the PEMFC’s efficiency can be optimized through a solid polymer electrolyte material in the PEM, considering the current most used polymer’s PEMFCs.
Background
The PEMFC has the means of creating clean and reliable energy. It is also very compact and quiet during its operation. Furthermore, the by-products produced as a result of the chemical reactions are environmentally friendly and can have a crucial effect in mitigating the rate of global warming (Brandon and Thompsett, 2005: 377).
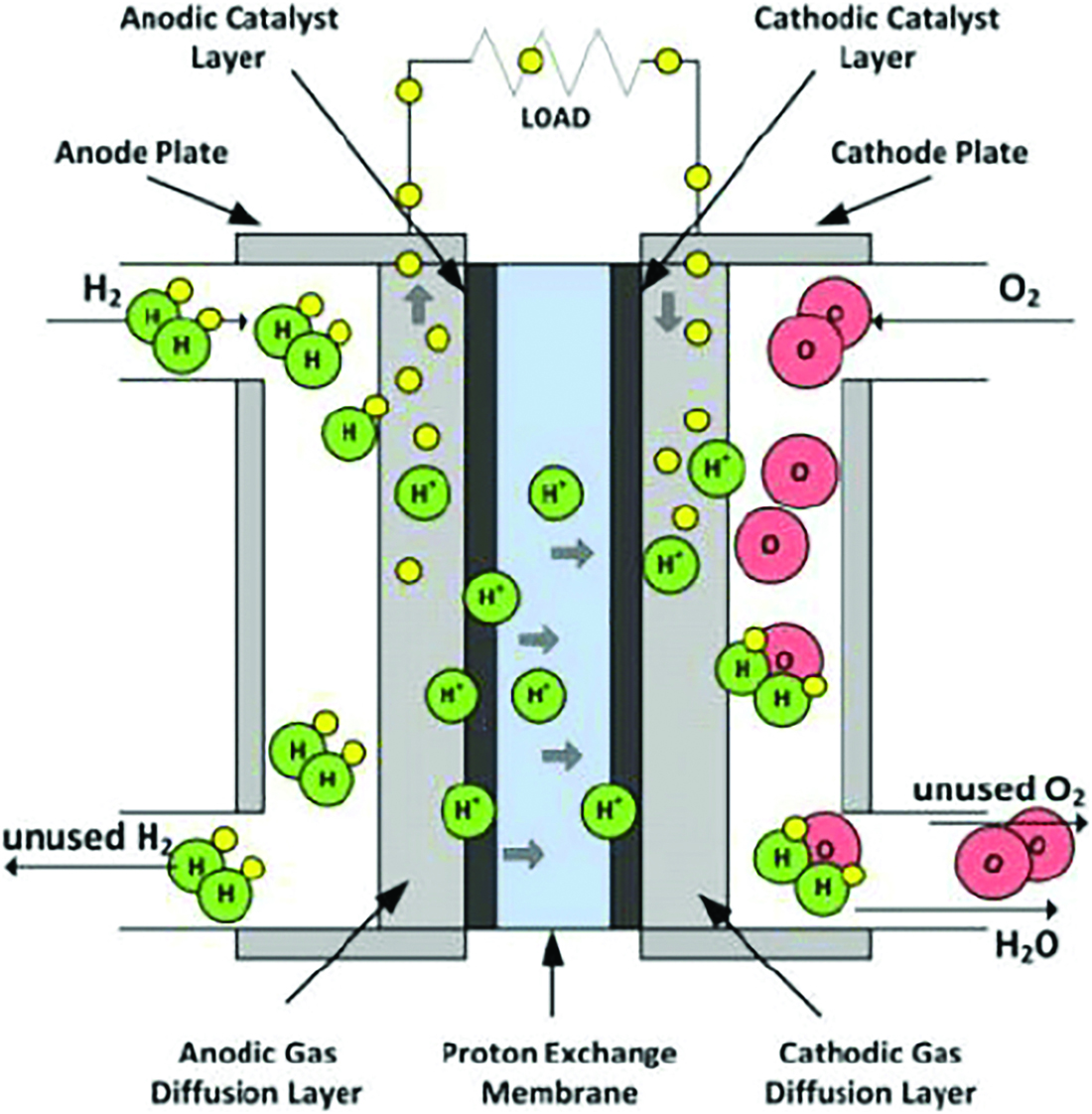
The main component within a PEMFC that creates energy is called the Membrane Electrode Assembly (MEA). It is composed of four key parts: solid polymer electrolyte membrane, the electrodes, the catalyst layers, and the gas diffusion layer. The electrodes consist of an anode and a cathode separated by the PEM. At the anode, hydrogen is chemically reduced, producing protons, electrons, and heat (Thompson). The reaction is accelerated with the aid of a platinum catalyst, given that the operation temperatures of a PEMFC do not allow for electrochemical reactions to occur at a rapid rate (Zhou and Chen, 2012).
Once the hydrogen is reduced, the protons migrate across the PEM, while the electrons enter the electrical circuit through the anode. The anode absorbs the electrons, which provides it with a negative charge. The electrons will travel from the negatively charged anode to the positively charged cathode. This migration produces voltage difference, which produces electricity. Concurrently, protons are traversing the PEM to the other side. On the cathode side, oxygen is present, hydrogen comes in through the membrane, and electrons exit the electrical circuit through the cathode. With these particles, the oxygen will be oxidized at the cathode to produce water.
Proton-Exchange Membrane
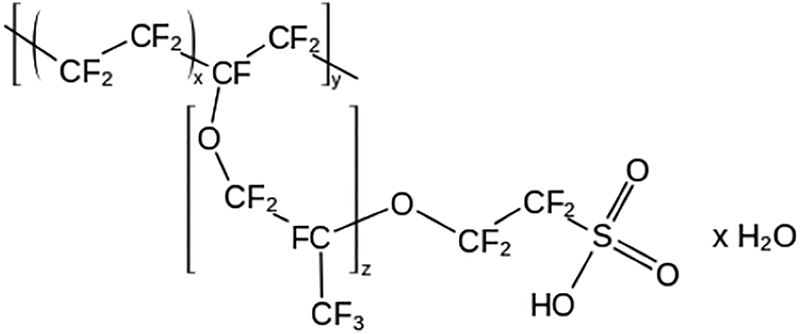
The PEM is an important component of the fuel cell. As previously mentioned, it allows protons to permeate to the cathode side. This characteristic is known as proton conductivity (Crofts, Antony, 1996). The PEM is unique compared to other fuel cell membranes, since it is a solid polymer electrolyte. This contrasts other fuel cells, which have liquid-based electrolytes composed of a fluorinated sulfonic acid polymer called Nafion (Brandon and Thompsett, 2005: 61). A benefit of using Nafion is that it can have excellent proton conductivity. In addition, the cathode on the other side of the membrane further aids in attracting the protons, increasing the PEMFC efficiency.
Nafion
Currently, one of the most common types of polymers for the electrolyte membrane is Nafion, a perfluorinated sulfonic acid ionomer (Rieke et al, 2001). As a polymer, Nafion is inhomogeneous, displaying hydrophobicity and hydrophilicity. Proton conduction is carried out by the hydrophilic, negatively charged sulfonate group, which contains one hydrogen, as seen in the next figure (Rieke et al., 2001). The group’s negative charge attracts protons, and, with the Grotthuss mechanism, they can jump across the sulfonic acid group donors until the protons reach the negatively charged cathode (Sun et al., 2015). With this characteristic, Nafion has proved to be effective, providing efficient proton conductivity and membrane lifetimes of over 60,000 hours (Hui San Thiam et al). In order to maintain this efficiency, Nafion has two important requirements.
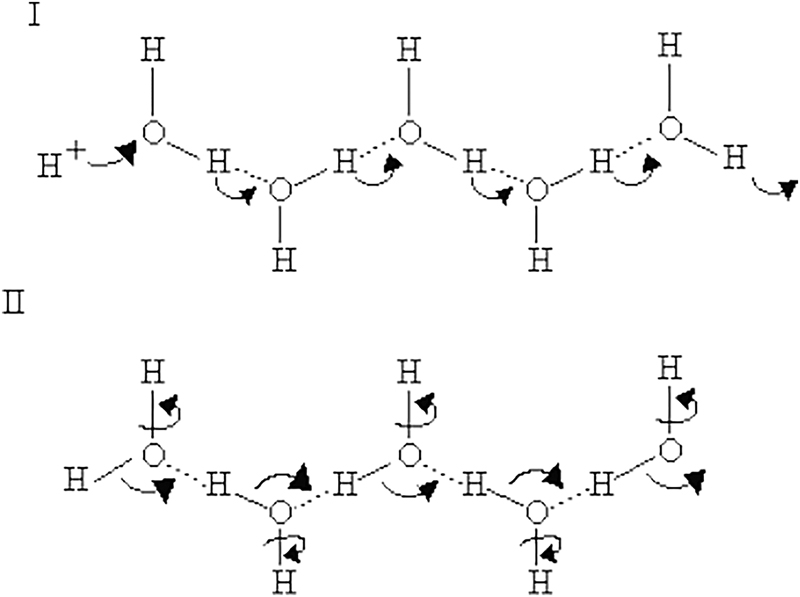
First, it is only able to conduct protons when hydrated. In low-humidity environments, its effectiveness rapidly declines despite the aforementioned Grotthuss mechanism that allows proton-jumping in hydrogen. This is attributed to the fact that under anhydrous conditions, it is more difficult for the essential hydrogen bonds to form on the sulfonic acid groups, hence making it less permeable (Brandon and Thompsett, 2005). Water lowers Nafion’s tightness, which helps its membrane structure to adapt to allow faster proton conductivity. Furthermore, water in itself is already effective as a proton conductor. Water molecules are also held together by hydrogen bonds, creating a network of hydrogen bonds that can be used by protons across the network, as seen in the next figure (Sun et al., 2015). In terms of the Nafion membrane, water molecules work in conjunction with the sulfonic acid groups. They create bridges between each of the sulfonic acid groups, allowing for protons to easily diffuse across the Nafion membrane. Thus, maintaining a consistent humidity within the PEMFC is extremely important, as having inadequate humidity will drastically impede proton conductivity.
Another important factor of a Nafion-based membrane is that temperature can also have an important effect on the membrane’s ability to conduct protons. In general, higher temperatures are desirable in the PEMFC since it allows for faster-moving particles. Ultimately, this leads to faster electrochemical reactions at both the anodes and the cathodes as well as faster proton conductivity (Zhou and Chen, 2012). However, a very high temperature is not achievable due to the limitations of the Nafion polymer. Nafion is dependent on liquid water for efficient proton conduction, which becomes an issue when higher temperatures decrease the humidity of the membrane. Thus, the Nafion membrane is limited to temperatures below 100°C (Zhou and Chen, 2012).
Overall, Nafion-based proton electrolyte membranes are limited by humidity and temperature. Manufacturers must consider this, meaning it becomes a difficult task to make the PEMFC more efficient. With other polymers that are not greatly limited by temperature and humidity, the PEMFC could become drastically more efficient. This investigation focuses primarily on temperature and its effects on the polymer.
Due to the aforementioned limitations of Nafion, a more effective replacement for the Nafion-based membrane is required. There is one type of polymer that can potentially replace Nafion-based proton electrolyte membranes: sulfonated aromatic polymers.
Sulfonated Aromatic Polymers
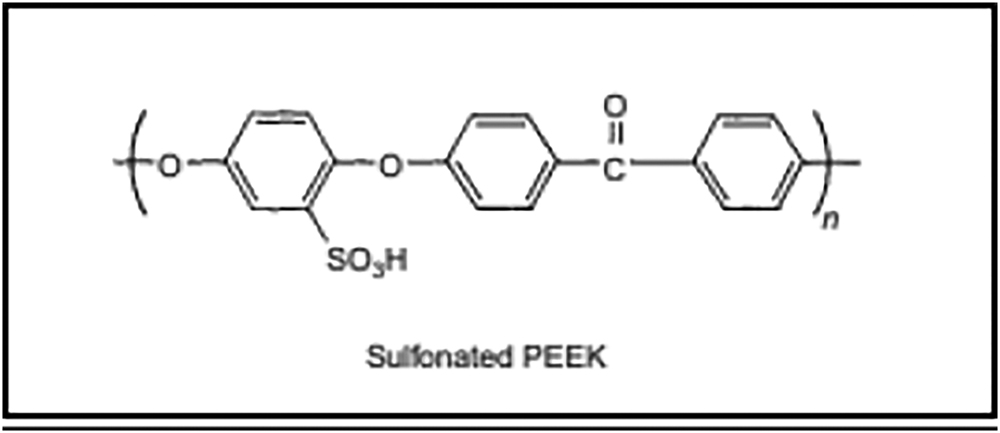
Sulfonated aromatic polymers are one important type of material that could effectively be used as a replacement for Nafion-based fuel cell membranes. These polymers consist of an aromatic polymer backbone and a proton-conducting polymer electrolyte. Aromatic polymers by themselves are composed primarily of benzene rings or aromatic heterocyclic rings (Liu et al, 2012). The linkages between the rings can be both regular and rigid or irregular, making them branched and more flexible. This characteristic of aromatic polymers allows them to have desirable thermal properties in a fuel cell (Liu et al., 2012). This same acidic property is able to be applied to sulfonic acids. Aromatic polymers can be sulfonated through the combination with a concentrated solution of acids, such as sulfuric acid, chlorosulfonic acid, pure or complexed sulfur trioxide, and acetyl sulfate (Brandon and Thompsett, 2005). Thus, an aromatic polymer that has great thermal properties now gains a sulfonic acid group, creating a sulfonated aromatic polymer. This sulfonated aromatic polymer, then, has the ability to conduct protons. This is important for proton electrolyte membranes, and the ability of sulfonated aromatic polymers to conduct protons opens the potential for them to replace Nafion. However, a further investigation and analysis needs to be done regarding their replaceability of Nafion-based membranes. One main polymer that could potentially replace Nafion is sulfonated poly (ether ether ketone). As a sulfonated aromatic polymer, it has a very strong backbone with benzene rings along with a sulfonic acid group that allows it to let protons pass through it with the Grotthuss mechanism.
Thermal Stability
Thermal stability of polymers is an extremely important part in creating an efficient proton electrolyte membrane; specifically, the ability to maintain structural integrity at high operating temperatures. Higher temperatures allow for a myriad of benefits: faster proton conductivity, low rates of catalyst poisoning, and little to no electrode flooding due to having water evaporate (Brandon and Thompsett, 2005: 382). Together, these properties prolong the life of a hydrogen fuel cell and give it more efficiency. This outcome is both cost-effective and efficient for the fuel cell.
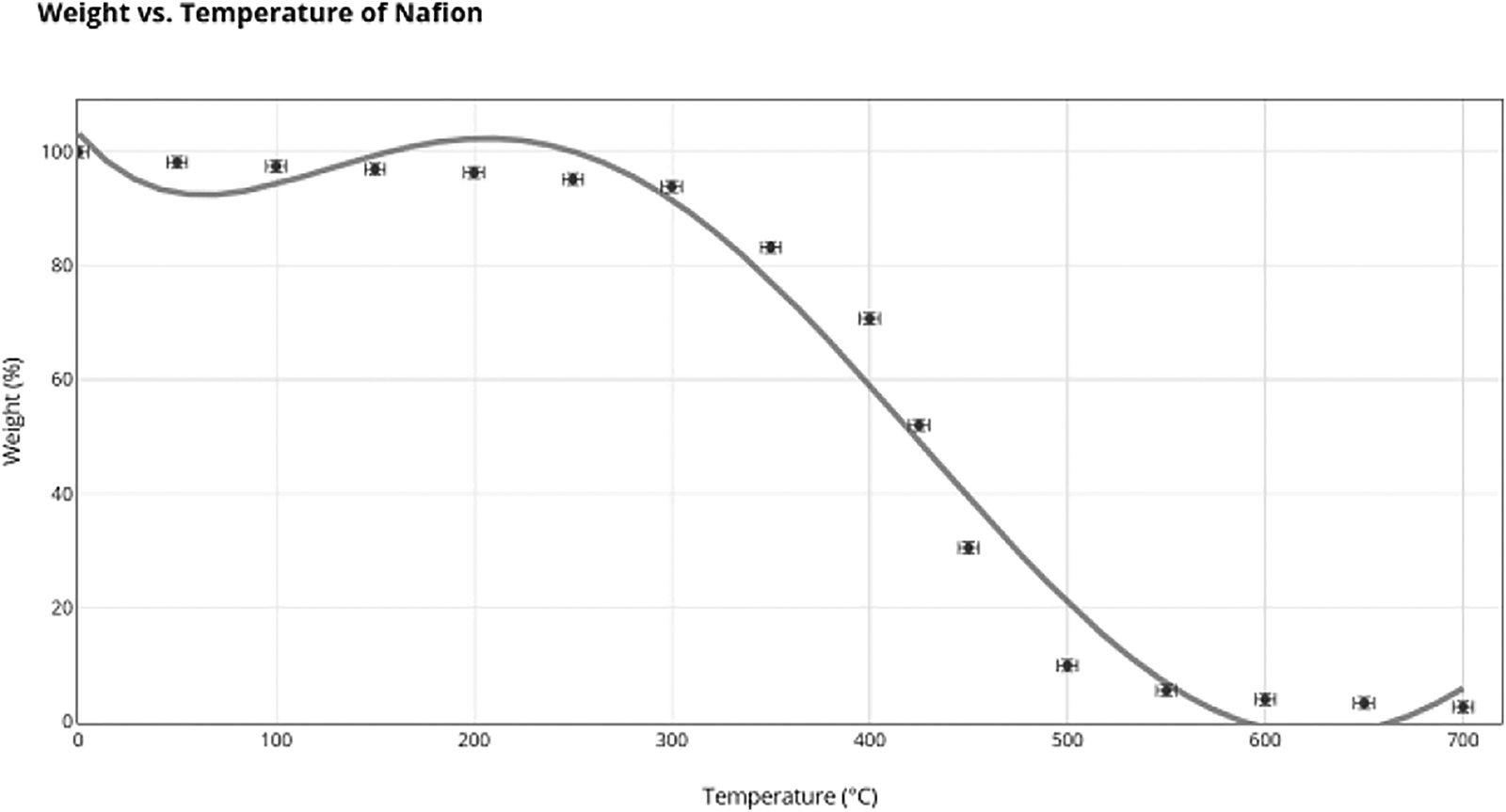
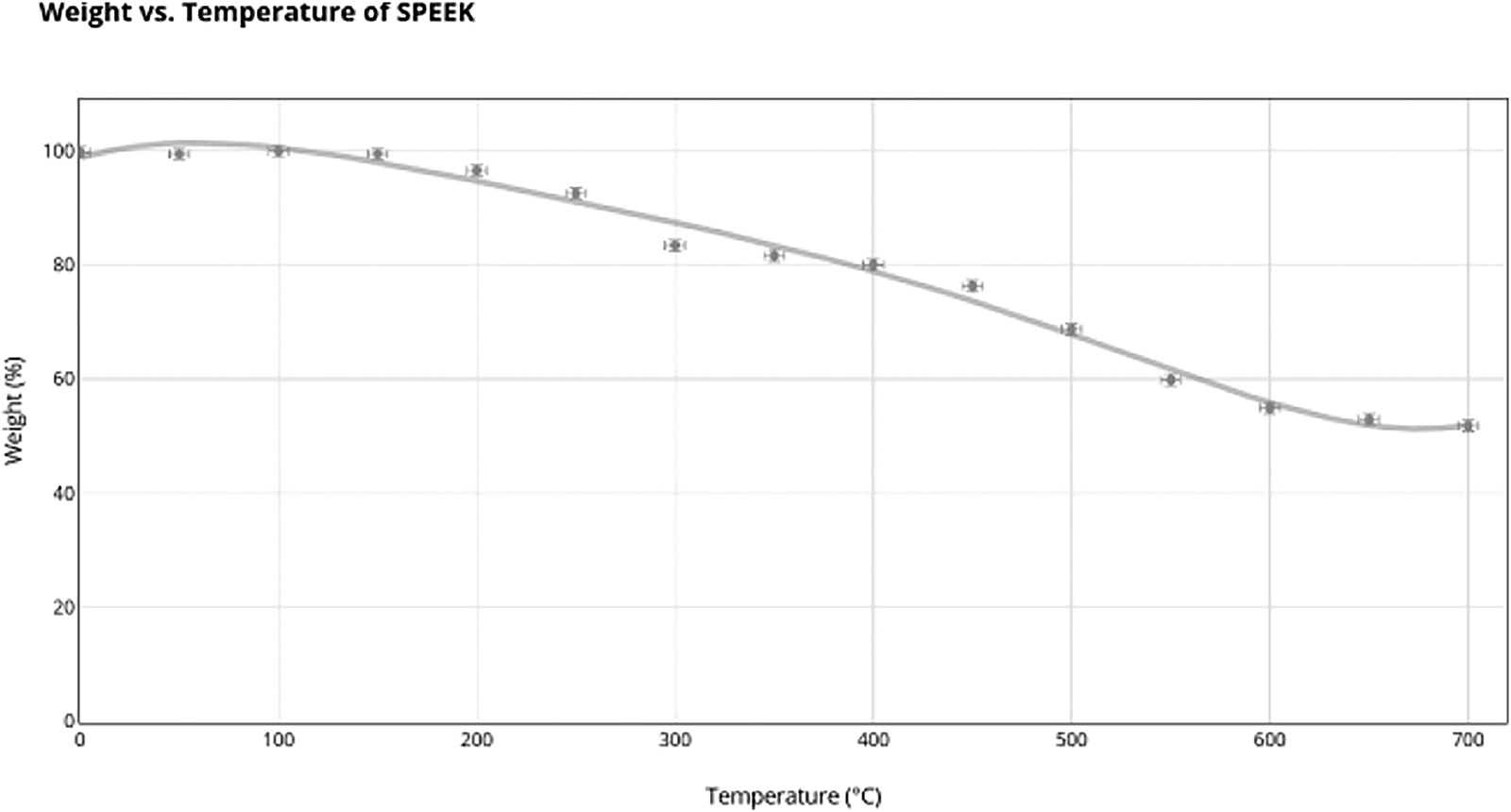
Thermal stability can be measured in many ways. One main way of measurement is through Thermogravimetric Analysis (TGA). TGA is used to measure the weight of molecules as a function of time or temperature. It can be used to measure a compound’s weight as it heats or cools; however, for Nafion and Sulfonated Poly(Ether Ether) Ketone (SPEEK), the temperature will be increasing as a strong thermal stability at high temperatures is needed from polymer electrolyte membranes in fuel cells. The focus of measuring temperature is to understand how it affects the weight loss in polymers. High temperatures make polymers increasingly unstable, leading to decomposition. Despite high temperatures being good, bad thermal stability signifies a lower lifespan for electrolyte membranes, which is a major disadvantage for an effective electrolyte membrane.
The TGA data will be translated into Derivative Thermogravimetric (DTG), which is a graph that shows how the TGA values are changing at different temperatures. DTG graphs are ones that represent the derivative of the TGA graphs. The peaks in DTG graphs let us understand the phase shifts of the polymer.
Weight Percentage Data
Nafion 117 |
SPEEK |
||
|---|---|---|---|
Temperature 5°C |
Percent Weight(%) 1 |
Temperature 1°C |
Percent Weight 1 |
0 |
99.9 |
0 |
99.7 |
50 |
98.1 |
50 |
99.4 |
100 |
97.4 |
100 |
99.9 |
150 |
96.9 |
150 |
99.4 |
200 |
96.3 |
200 |
96.5 |
250 |
95.1 |
250 |
92.5 |
300 |
93.8 |
300 |
83.4 |
350 |
83.2 |
350 |
81.6 |
400 |
70.7 |
400 |
80 |
450 |
30.5 |
450 |
76.3 |
500 |
9.87 |
500 |
68.7 |
550 |
5.49 |
550 |
59.8 |
600 |
3.93 |
600 |
54.9 |
650 |
3.29 |
650 |
52.9 |
700 |
2.6 |
700 |
51.8 |
The Dagra Blue Leaf software will be used to extract raw values present in the graph. The data for both Nafion and SPEEK will be analyzed in intervals of , which provides a cohesive graph of how they fluctuate throughout the environments with increasing temperatures. The uncertainties from the literature data will be utilized.
After this extraction of TGA data, the derivative of the curve of best fit will be created. This allows a more in-depth analysis of where the different weight loss shifts occur within Nafion and SPEEK at high temperatures.
Nafion data has been taken from the source publication study, “The quintuple-shape memory effect in electrospun nanofiber membranes” (Zhang et al., 2013).
SPEEK data has been taken from the source publication study, “Electrospun multifunctional sulfonated carbon nanofibers for design…” (Li et al., 2017).
Quantitative Interpretation for Thermal Data
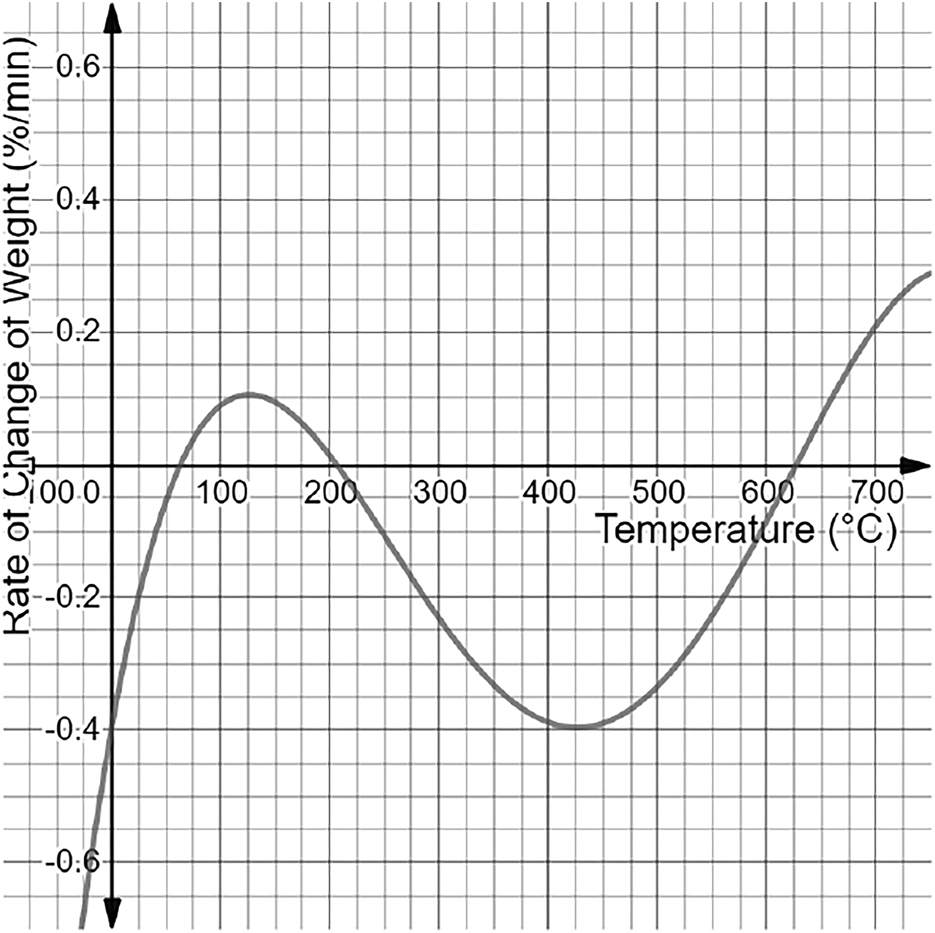
According to Nafion’s TGA graph, Nafion’s final weight at is 2.6% of its initial mass. Further, the DTG graph points at which it equals 0, representing the peaks of Nafion. The two low peaks of Nafion are at temperatures of and , while the single high peak is at a temperature of . Nafion’s derivative TGA indicates a one high peak at , with a rate of weight change of 0.105% and one low peak at with a rate of weight change of −0.4%.
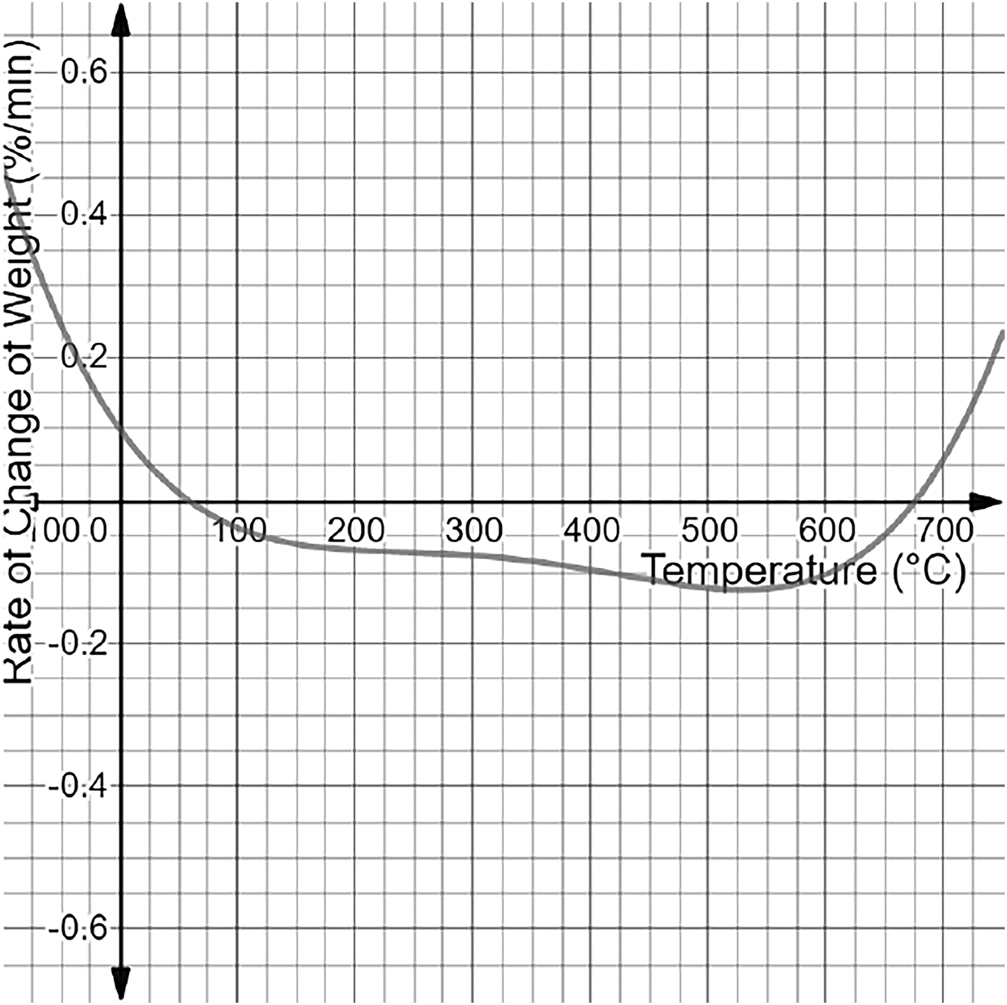
Shifting to SPEEK’s final weight percentage at is 51.8%. Its corresponding DTG graph showcases that the TGA graph of SPEEK contains a maximum peak at and a minimum peak at . Furthermore, the peak of the DGA graph is at a temperature of with a rate of weight change of −0.125%.
Thermal Data Analysis
The DGA graph provides a range of important information to understand the TGA graph and the thermal stabilities of Nafion and SPEEK. Initially, it can be seen from the TGA data that Nafion’s final weight percentage is 2.6% and SPEEK’s is 51.8%. However, this does not provide information about the usefulness of a polymer’s thermal properties in a fuel cell membrane, as even high temperatures do not operate at temperatures of . Thus, a more in-depth understanding can be gained by paying more attention to the DGA data. The peaks of the polymers in terms of weight loss were calculated using the derivative TGA graphs. We observe that Nafion exhibits two close peaks at and . These peaks are very similar and have a very small range of weight percentage of 92–100%; they do not indicate any major molecular phase shifts. SPEEK has an initial maximum of 58.663, which from the initial temperature has ranges of 98–100%. Though these initial peaks don’t showcase phase shifts, they showcase that Nafion and SPEEK are thermodynamically very similar at the initial temperature increases.
The following peaks calculated by the derivative graph occurs at , while SPEEK exhibits its minimum peak at . SPEEK’s last minimum peak occurs at higher than Nafion’s, indicating two things. SPEEK is potentially more stable as its peak occurs later than Nafion’s, showcasing that more temperature is required and, thus, more energy is required to break down bonds within SPEEK.
However, a stronger conclusion can be built with the peaks of the DGA graphs themselves. The peaks of the DGA graphs indicate inflection points on the TGA graphs. These inflection points will have the greatest rate of change exhibited by the polymer. A great rate of change is a primary example of a phase shift in a polymer at a certain temperature. Phase shifts occur when a certain molecular part of the polymer starts to rapidly decompose under a specific temperature, and thus, the inflection points locate the same. The Nafion DGA graph indicates a main peak of −0.396 at a temperature of . Therefore, Nafion has a large phase shift in molecular decomposition with a very fast rate of decomposition. SPEEK indicated a phase shift at , which occurred much later and had a much lower rate than Nafion at −0.125. SPEEK reaches the greatest weight loss% at later than Nafion, and it also has a much lower overall value. Molecularly, this can be attributed to the fact that SPEEK is more regular than Nafion and thus forms a tighter regular polymolecular structure. While the data showcases that Nafion exhibits its first weight loss phase at based on the TGA peak compared to that of SPEEK earlier at , SPEEK demonstrates a much lower weight loss rate overall and enters its shift in phase much later than Nafion. Therefore, the aromatic backbone gives SPEEK more thermal stability.
The final thing that needs to be taken into account is the temperature range in which the sulfonic acid groups decompose in both Nafion and SPEEK. Despite established data that SPEEK is more thermally stable from 0–, sulfonic acid groups primarily provide both Nafion and SPEEK with their proton-conducting properties; their loss directly impacts fuel cell efficiency. Thus, they need to be taken into the greatest account. The initial DGA peaks showcased inflection points, which showcased the moment in which both Nafion and SPEEK experienced phases shifts in molecular decomposition. The decomposition occurring prior to this phase shift would be that of the sulfonic acid groups. Since these groups have weaker linkages compared to those of the backbones in Nafion and SPEEK, they decompose quicker. It may appear that the sulfonic groups are decreasing from 0°C to in Nafion and from 0°C to in SPEEK, but the rate of change of both these areas is very low. The rate thus indicates that the decomposition must be the water molecules that have been absorbed. Nafion has hydrophilic areas, and SPEEK is nearly fully hydrophilic, making them eligible to absorb water. Thus, this low decomposition can be fully attributed to water loss. After that, another drop in the rate of change occurs. As stated, the inflection point of Nafion is at , and SPEEK’s is at . The inflection point is based on a phase shift, and, due to the chemical changes that occurred, SPEEK’s decomposition must occur in the first phase of weight loss. This is due to the sulfonic acid group’s more unstable nature, causing it to decompose at earlier temperatures. The most crucial concept is that Nafion is moving into the second phase much earlier, thereby losing most of its sulfonic acid groups earlier than SPEEK while also at a faster rate of −0.4 compared to 0.125 for SPEEK. This overall makes Nafion more thermodynamically stable than SPEEK.
Nafion could still be utilized at lower temperatures, given its lack of weight loss and low rate of change in weight, indicating its stability. However, after temperatures of , SPEEK retains more thermodynamic stability, and Nafion begins to decompose more rapidly.
Proton Conductivity
The proton conductivities at increasing temperatures must also be analyzed. The proton conductivity of a polymer is the ability of the polymer to transport hydrogen cations from the anode to the cathode. The faster this occurs, the faster more hydrogen can enter the initial anode area. If proton conductivity is slow, then, kinetically, it will be more difficult for the hydrogen to oxidize and create protons and electrons. Proton conductivity in itself is temperature dependent, and, based on the molecular structure of the membrane, temperature can further exasperate the rate of it.
Proton conductivities can be measured in a myriad of ways. Direct data that compares proton conductivity to temperature, for example, can be utilized. However, despite its importance, more analysis would be needed. One way to create a linear correlation between temperature and proton conductivity employs the Arrhenius equation (proton conductivity). This equation provides a linear relationship and insight into the activation energy and efficiency of proton conduction.
Converting Arrhenius Equation
Where
The natural log of each side can be taken.
Taking the natural log and graphing it against the reciprocal of temperature produce a linear graph, all else constant. As this is a line, a direct relationship between proton conductivity and temperature is apparent. The activation energy will also be expressed as the slope of this line.
Proton Conductivity Data
Nafion |
SPEEK |
||
|---|---|---|---|
Temperature ± 5oC |
σ ± 0.01 |
Temperature ± 1oC |
σ ± 0.01 |
20 |
0.0402 |
20 |
0.0245 |
30 |
0.0483 |
30 |
0.0253 |
40 |
0.0562 |
40 |
0.0284 |
50 |
0.0633 |
50 |
0.0315 |
60 |
0.0704 |
60 |
0.0363 |
70 |
0.0771 |
70 |
0.0392 |
80 |
0.0841 |
80 |
0.0421 |
90 |
0.0866 |
90 |
0.0431 |
100 |
0.0878 |
100 |
0.0441 |
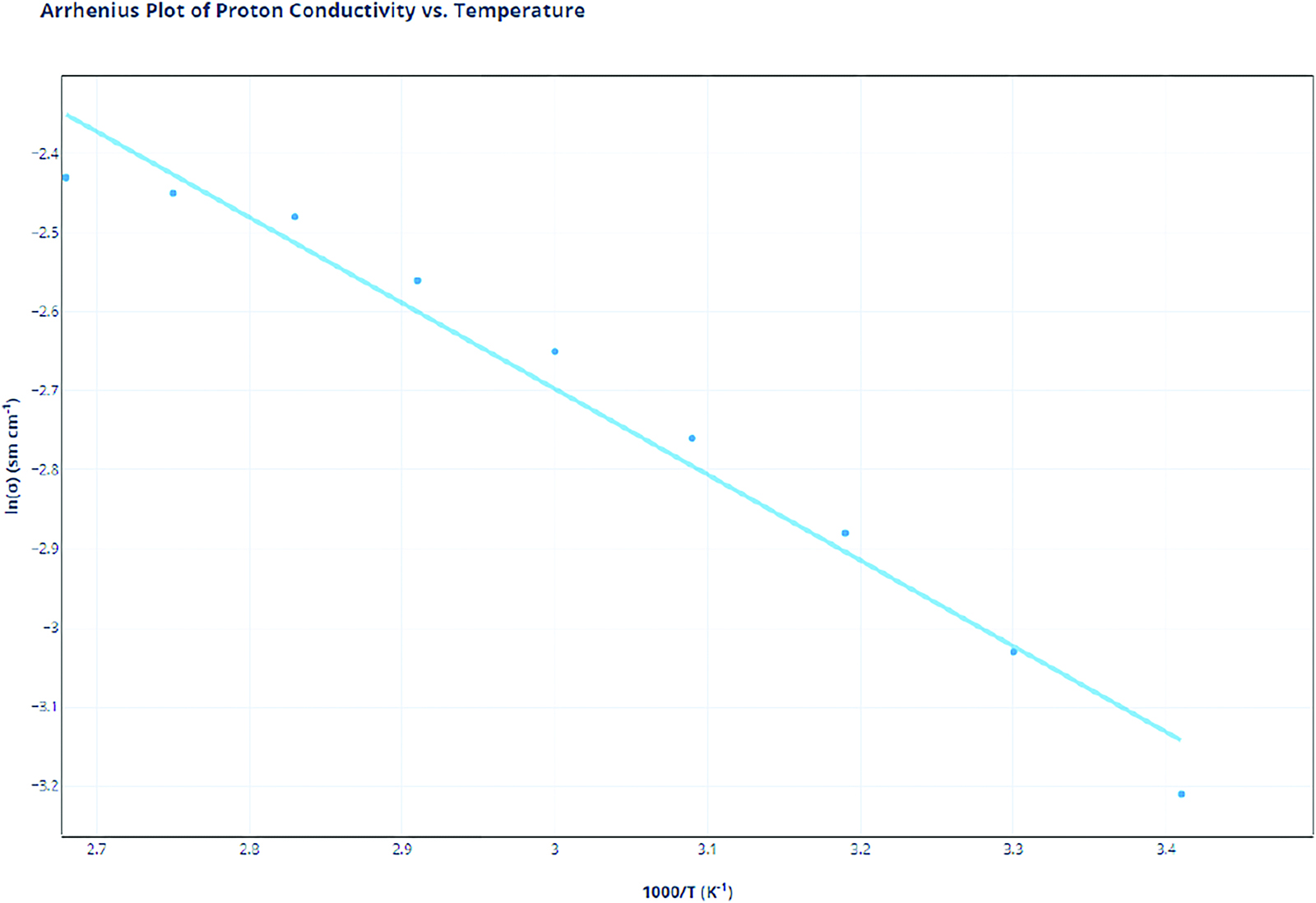
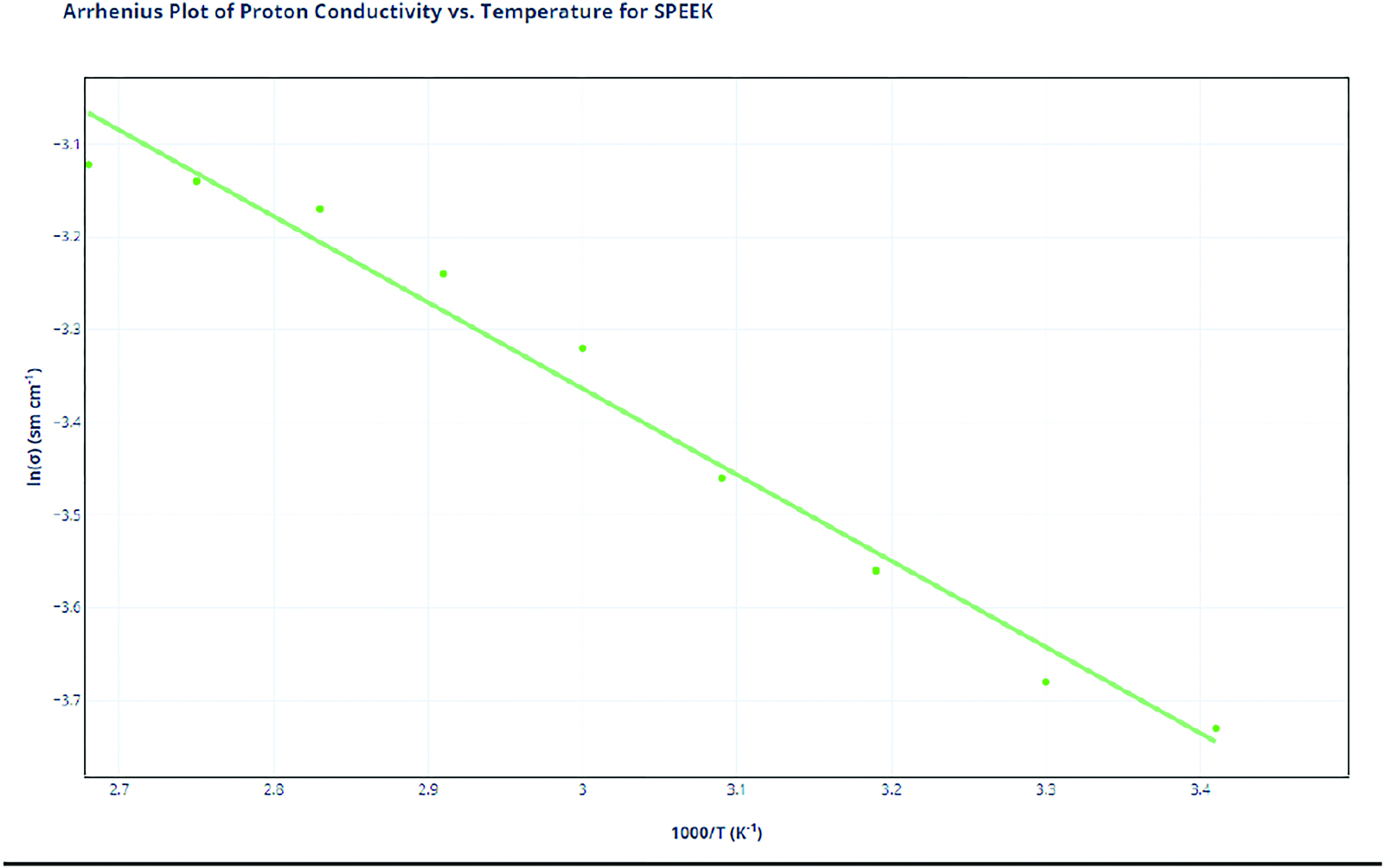
With this data, two calculations need to be tabulated. First, the reciprocal of the temperature (in Kelvin) multiplied by 1000 is required, as this will provide the x values for the Arrhenius plot. Next, the natural logarithm of the proton conductivity will be taken. Blue Dagra software is used to analyze the literature data graphs. The uncertainties will be provided from the literature data and any appropriate calculations that may affect those uncertainties. Nafion was procured from the paper: “Preparation of Poly(Styrenesulfonic Acid) Grafted Nafion with a Nafion-Initiated Atom Transfer Radical Polymerization for Proton Exchange Membranes” (Peng et al., 2017). SPEEK data was procured from “Preparation and Characterization of Carbon Molecular Sieve (CMS)/SPEEK Bilayer Membranes and SPEEK/Polyimide (PI) Blend Membranes for Direct Alcohols Fuel Cell (DAFC) Performance (Maab et al., 2013).
Nafion |
SPEEK |
||
|---|---|---|---|
1000/T(K) |
ln(σ) |
1000/T |
ln(σ) |
3.41 |
−3.21 |
3.41 |
−3.73 |
3.3 |
−3.03 |
3.3 |
−3.68 |
3.19 |
−2.88 |
3.19 |
−3.56 |
3.09 |
−2.76 |
3.09 |
−3.46 |
3 |
−2.65 |
3 |
−3.32 |
2.91 |
−2.56 |
2.91 |
−3.24 |
2.83 |
−2.48 |
2.83 |
−3.17 |
2.75 |
−2.45 |
2.75 |
−3.14 |
2.68 |
−2.43 |
2.68 |
−3.12 |
Proton Conductivity Data Analysis
The Arrhenius plot of the proton conductivities showcases varying degrees of overall proton conductivity at different temperatures. In addition, it provides insight into the capabilities of Nafion and SPEEK as temperature starts to increase. Based solely on the overall line for both SPEEK and Nafion, SPEEK’s overall Arrhenius plot is lower than Nafion’s plot. We can conclude that Nafion has a higher proton conductivity in comparison to SPEEK.
However, the rate of changes also provides important information. Nafion’s rate of change is of −0.1.08 compared to SPEEK’s slope of −0.929. Both rates of change are decreasing because both Nafion and SPEEK operate efficiently in an excess of water. This outcome can be attributed to the Grotthuss mechanism. As temperature increases, water starts evaporating, disrupting the mechanism and preventing efficient proton conductivity. SPEEK’s rate of change is lower in comparison to that of Nafion, indicating that it is less dependent on water in comparison to Nafion as temperatures increase. While the temperature gets negatively impacted, SPEEK’s overall reliance on water is much less due to the fact that SPEEK is much more regular than Nafion. Its regular structure and aromatic rings allow the SPEEK molecules to be physically closer, indicating that the sulfonic acid groups are in much higher concentration per mole of SPEEK compared to Nafion. More sulfonic acid groups lead to higher overall conductivity and less reliance on water. Thus, as temperature rises and water volume starts to decrease in the membrane, Nafion showcases a stark decrease in proton conductivity, while SPEEK slowly decreases but maintains consistency overall.
Conclusion
Based on the thermal analysis and the proton conductivities, SPEEK showcased more stability at high temperatures compared to Nafion, while Nafion showcased stronger proton conductivities. However, despite Nafion’s higher proton conductivity, SPEEK showcases much less degradation, an overall lower activation energy, and a decrease in proton conductivity at higher temperatures. Nafion, however, experiences drastic decreases. SPEEK exhibits a higher level of proton conductivity and surpasses Nafion’s temperature limit of 100°C due to its densely packed sulfonic acid group. This structural characteristic enables SPEEK to maintain its proton conductivity without experiencing significant losses even at elevated temperatures. Nafion’s overall superiority is essentially surpassed by SPEEK. Thus, SPEEK is, to a good extent, a suitable alternative for Nafion if it is being utilized at temperatures higher than 100°C where Nafion starts to diminish in effectiveness.
Our analysis did not consider other factors, including relative humidity, ion-exchange capacity, and sulfonation levels. These are also important factors that can determine more information about proton conductivity. They will also require their own investigations, potentially shedding more insight into the effectiveness of SPEEK and its suitability as a replacement for Nafion in the PEM. Furthermore, combining SPEEK with another polymer that is effective in proton conduction should be analyzed further. Nafion itself is thermally stable as demonstrated; however, combining it with another polymer to create a thermally stable co-polymer with high proton conductivity could potentially allow for an important comparison between the new copolymer and Nafion. Additional factors that affect proton conductivity need to be considered for this analysis.
References
Brandon, N. P., & Thompsett, D. (2005). Fuel Cells Compendium. Elsevier.
Crofts, Antony (1996). “Lecture 12.” Lecture 12: Proton Conduction, Proton Channels, Proton Wells, www.life.illinois.edu/crofts/bioph354/lect12.html.www.life.illinois.edu/crofts/bioph354/lect12.html
Li, X., Lu, Y., Ren, X., Wang, L., & Zhang, H. (2017). Electrospun multifunctional sulfonated carbon nanofibers for design and fabrication of SPEEK composite proton exchange membranes for direct methanol fuel cell application. ResearchGate. https://www.researchgate.net/publication/314648271_Electrospun_multifunctional_sulfonated_carbon_nanofibers_for_design_and_fabrication_of_SPEEK_composite_proton_exchange_membranes_for_direct_methanol_fuel_cell_applicationhttps://www.researchgate.net/publication/314648271_Electrospun_multifunctional_sulfonated_carbon_nanofibers_for_design_and_fabrication_of_SPEEK_composite_proton_exchange_membranes_for_direct_methanol_fuel_cell_application
Liu, B., & Guiver, M. D. (2012). Proton conductivity of aromatic polymers. In Solid State Proton Conductors (pp. 331–369). Wiley.
Maab, H., & Shishatskly, S. (2013). Preparation and characterization of carbon molecular sieve (CMS)/SPEEK bilayer membranes and SPEEK/polyimide (PI) blend membranes for direct alcohols fuel cell (DAFC) performance. ResearchGate. https://www.researchgate.net/publication/260276154_Preparation_and_characterization_of_carbon_molecular_sieve_CMS_SPEEK_bilayer_membranes_and_SPEEK_polyimide_PI_blend_membranes_for_direct_alcohols_fuel_cell_DAFC_performancehttps://www.researchgate.net/publication/260276154_Preparation_and_characterization_of_carbon_molecular_sieve_CMS_SPEEK_bilayer_membranes_and_SPEEK_polyimide_PI_blend_membranes_for_direct_alcohols_fuel_cell_DAFC_performance
Parts of a fuel cell. Energy.gov. (n.d.). Retrieved April 30, 2023, from https://www.energy.gov/eere/fuelcells/parts-fuel-cellhttps://www.energy.gov/eere/fuelcells/parts-fuel-cell
Peng, K.-J., Yang, Y.-H., Lee, C.-Y., & Lin, K.-C. (2017). Preparation of Poly(Styrenesulfonic Acid) Grafted Nafion with a Nafion-Initiated Atom Transfer Radical Polymerization for Proton Exchange Membranes. Semantic Scholar. https://www.semanticscholar.org/paper/Preparation-of-Poly(Styrenesulfonic-Acid)-Grafted-Peng-Yang/b1ce77213937b0735142bdc1868d8617191f487dhttps://www.semanticscholar.org/paper/Preparation-of-Poly(Styrenesulfonic-Acid)-Grafted-Peng-Yang/b1ce77213937b0735142bdc1868d8617191f487d
“Proton Conductivity.” (n.d.). In ScienceDirect Topics. Retrieved April 30, 2023, from https://www.sciencedirect.com/topics/engineering/proton-conductivityhttps://www.sciencedirect.com/topics/engineering/proton-conductivity
Zhou, Su, and Fengxiang Chen. “PEMFC System Modeling and Control.” Fuel Cell Engineering, 2012, pp. 197–263, https://doi.org/10.1016/b978-0-12-386874-9.00007-5.https://doi.org/10.1016/b978-0-12-386874-9.00007-5
Rieke, P. C., & Vanderborgh, N. E. (2001). Temperature dependence of water content and proton conductivity in polyperfluorosulfonic acid membranes. Journal of Membrane Science, 192(1–2), 169–179. https://doi.org/10.1016/S0376-7388(00)85014-0https://doi.org/10.1016/S0376-7388(00)85014-0
Sun, H., Li, H., & Li, B. (2015). A molecular dynamic simulation of hydrated proton transfer in perfluorosulfonate ionomer membranes (Nafion 117). Journal of Chemistry, 2015, 1–9. https://doi.org/10.1155/2015/169680https://doi.org/10.1155/2015/169680
Thompson, E. L. (n.d.). Electron Microscope Analysis of Low Temperature Damage in PEM Fuel Cells. Retrieved April 30, 2023, from http://www2.optics.rochester.edu/workgroups/chttp://www2.optics.rochester.edu/workgroups/c
Zhang, F., Zhang, Z., Liu, Y., Lu, H., & Leng, J. (2013). The quintuple-shape memory effect in electrospun nanofiber membranes. Smart Materials and Structures, 22(8), 085020.
Hui San Thiam, et al. “Nafion-Based Membrane.” Encyclopedia, 20 May 2022, encyclopedia.pub/entry/22977#:~:text=As%20a%20result%2C%20Nafion%20may,an%20outstanding%20lifespan%20for%20Nafion.encyclopedia.pub/entry/22977#:~:text=As%20a%20result%2C%20Nafion%20may,an%20outstanding%20lifespan%20for%20Nafion