Introduction
Despite being one of the most researched and preventable chronic health conditions, cardiovascular disease (CVD) is the leading cause of death in the United States (US) and globally. CVD describes a range of conditions impacting the heart and vascular system, with the most common presentations being angina, myocardial infarction, and heart failure (CDC, 2018; Lopez et al., 2006; Flora & Nayak, 2019). The number of CVD cases has nearly doubled from 271 million in 1990 to 523 million in 2019, and the number of CVD-related deaths has also increased from 12.1 million in 1990 to 18.6 million in 2019 (Roth et al., 2020). In addition to contributing to morbidity and mortality globally, CVD has placed an extreme burden on healthcare systems and societies. In 2010, the global cost of CVD was an estimated $865 billion and is estimated to rise to $1.05 trillion by 2030. Around half of the monetary loss is due to direct healthcare costs, and the other half is productivity loss from not working, disability, or premature death (World Economic Forum, 2011). Therefore, the development of diagnostic tools for early identification of individuals at risk of developing CVD is crucial for timely diagnosis and intervention.
The cardiovascular system is made of the heart and blood vessels. CVD refers to the wide range of states of disease within this system (Farley et al., 2012). Although CVD can originate from a variety of elements, atherosclerosis, the obstructive deposition of fatty substances inside the arteries, is a primary cause of CVD (Libby et al., 2011).
The majority of CVD cases can be grouped into 4 categories:
Coronary artery disease (CAD): Plaque buildup in the arteries that supply blood to the heart, which leads to narrowing or blockage in these vessels. Results in angina, MI, and/or heart failure.
Cerebrovascular disease (CVD): Plaque buildup in the arteries that supply blood to the brain, which leads to narrowing or blockage in these vessels. Includes stroke and transient ischemic attack (TIA).
Peripheral artery disease (PAD): Plaque buildup in the peripheral arteries (located in the legs and arms), which leads to narrowing or blockage in these vessels.
Aortic atherosclerosis: Plaque buildup in the aorta, which leads to narrowing or blockage in this vessel, resulting in diminished blood flow and weakening of the aorta. Includes thoracic and abdominal aneurysms (Benjamin et al., 2018).
Multiple microscopic biological phenomena, including dyslipidemia, inflammation, and endothelial dysfunction, lead to the formation of fatty streaks (Davies et al., 1988), characteristic of atherosclerosis. Well-known risk factors contributing to these biomarkers of CVD are modifiable and include hypertension (HTN), dyslipidemia, diabetes mellitus (DM), smoking and secondhand smoke exposure, obesity, unhealthy diet, and sedentary lifestyle (CDC, 2022; Carey et al., 2021; Wilson et al., 1998; Alloubani, 2021; Jung et al., 2022). HTN is defined as a systolic blood pressure (SBP) ≥130mmHg and/or a diastolic blood pressure (DBP) >80mmHg and is the leading risk factor for CVD morbidity and mortality (Carey et al., 2021; Iqbal & Jamal, 2022). Dyslipidemia is defined as abnormal levels of lipids and/or lipoproteins in the blood, including elevated total cholesterol (TC ≥200 mg/dL), low-density lipoprotein cholesterol (LDL-C >100 mg/dL), triglyceride (TG >150 mg/dL), and low high-density lipoprotein cholesterol (HDL-C <40 mg/dL) (Alloubani, 2021, Jung et al., 2022). In the US, the number of adults with HTN is about 116 million and globally is about 4 billion (Roth et al., 2020). In the US, around 38% of adults have total cholesterol ≥200 mg/dL, 28% of adults have LDL ≥130 mg/dL, 21% of adults have TG ≥150 mg/dL, and 17% of adults have HDL <40 mg/dL (Tsao et al., 2022). Both HTN and dyslipidemia have been widely researched and have much data pointing toward their responsibility in the development of CVD, but studies have also demonstrated their insufficiency in independently producing all cases of CVD (Ridker et al., 2004; Libby et al., 2002; Khot, 2003; Johnson et al., 2018). Inflammation has emerged as another factor in the development of CVD (Sorriento & Iaccarino, 2019; Chen et al., 2022). Inflammation is the immune system’s reaction to dangerous substances, such as pathogens, damaged cells, toxins, or radiation. This process is crucial for maintaining health as it removes damaging entities and promotes healing. During acute inflammation, the immune system works to mitigate injury or infection. However, if inflammation becomes chronic, it can wreak havoc on the body and is known to contribute to a variety of debilitating health conditions, such as rheumatoid arthritis, lupus, periodontitis, and atopic dermatitis (Chen et al., 2017; Sorriento & Iaccarino, 2019). Past research has also shown a strong correlation between chronic inflammatory states and risk of developing atherosclerotic plaque and CVD (Koosha et al., 2020; Ferrucci & Fabbri, 2018). In blood vessels, inflammation occurs in response to vessel injury, oxidation of serum lipids, and infection. HTN damages vessel endothelium, prompting an inflammatory response. In response to endothelial damage, leukocytes bind monocytes to the affected site. Dyslipidemia increases the risk of serum lipids getting stuck behind the endothelium and becoming oxidized. This triggers a stronger inflammatory response because monocytes that interact with these oxidized particles are more likely to stay bound to the endothelium. Furthermore, the monocytes can transform into macrophages and foam cells, which are precursors in the development of plaque. Other risk factors, including DM, smoking and secondhand smoke exposure, obesity, unhealthy diet, and sedentary lifestyle, amplify the harmful inflammatory effects of HTN and elevated lipids (Willerson & Ridker, 2004; Martinez-Quinones et al., 2018; Steinberg, 2009; Rhoads & Major, 2018; Tabas et al., 2007). Systemic inflammation can be measured by a variety of blood-based biomarkers, with the most prominent being C-reactive protein (CRP), which is strongly associated with CVD (Koosha et al., 2020; Ferrucci & Fabbri, 2018; Castro et al., 2018).
Although there is prior research examining the link between inflammation and CVD risk, there is limited research on the co-occurrence of inflammation, HTN, and dyslipidemia and resultant CVD risk. There remains an important question: Does an individual need all the 3 factors to develop CVD? Present research also includes samples that are often small and not directed toward geriatric populations, which is important considering older individuals are the primary group affected by CVD. In this present study, we will determine if having all 3 risk factors—high inflammation, hypertension, and dyslipidemia—correlates with a greater risk of CVD, as opposed to having 0–1 or 2 of these risk factors, using data from the Health and Retirement Study (HRS), a nationally representative, longitudinal study of adults over 50 years. We will describe the demographics (age, sex, race) and risk factors (obesity, smoking status, DM, HTN, dyslipidemia, inflammation) of HRS participants with CVD status. In addition, we will describe the demographics (age, sex, race) and risk factors (obesity, smoking status, DM, HTN, dyslipidemia, inflammation) of HRS participants with risk factor status. Finally, we will examine the association between risk factor status and CVD status, controlling for age, sex, race, obesity, smoking status, and DM.
Methods
Study Design and Data Sources
Data for this study come from HRS, an ongoing nationally representative panel study that surveys around 20,000 people older than age 50 in the US every 2 years. This study is supported by the National Institute on Aging (NIA) and Social Security Administration (SSA) and is coordinated by the University of Michigan Institute for Social Research (ISR). The design and methodology of HRS have been thoroughly outlined elsewhere (Sonnega et al., 2014; Karp, 2007; Juster & Suzman, 1993). In this study, we carried out a cross-sectional analysis examining the association between CVD prevalence and clinical risk factors for CVD using data from 2016 (Wave 13). Of the 9,850 participants with biomarker data available at this wave, we excluded individuals missing data on BP, lipids, and CRP, as well as covariates including age, sex, race/ethnicity, DM or hyperglycemia, smoking, and obesity to obtain a final sample of 1,527 participants.
Exposure
CVD risk score was generated from 3 clinical conditions known to contribute to CVD: HTN, dyslipidemia, and elevated CRP. HTN status was obtained from HRS self-reported health data on the basis of a healthcare provider’s diagnosis. LDL-C was calculated in serum specimens having a triglyceride value <400 mg/dL using the formula of Friedewald LDL-C = TC − HDL-C − TG/5.0. HDL-C was measured directly in serum using the Roche HDL-C 3rd-generation direct method (Roche Diagnostics, Indianapolis, IN) on a Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics Corporation). TG was measured in serum using an enzymatic TG Reagent (Roche Diagnostics, Indianapolis, IN) on a Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics Corporation). Dyslipidemia was categorized as LDL-C >130 mg/dL, TG >150 mg/dL, or HDL-C <50 mg/dL for women and <40 mg/dL for men, based on widely recognized clinical reference ranges (Martin & Cardoso, 2021). CRP was measured in serum using a latex-particle enhanced immunoturbidimetric assay kit (Roche Diagnostics, Indianapolis, IN 46250) and read on the Roche COBAS 6000 Chemistry analyzer (Roche Diagnostics). High CRP was categorized as >3 mg/L, based on the widely recognized clinical reference range (HRS, 2016; Adukauskienė et al., 2016). Values of continuous CRP were log transformed for descriptive statistics.
Low risk was defined as having 0–1 diagnoses of HTN, high CRP, or dyslipidemia; medium risk was defined as having 2 of these factors; and high risk was defined as having all 3 factors.
Outcome
Self-reported health data of a healthcare provider’s diagnosis of heart attack, coronary heart disease, angina, congestive heart failure or other heart problems, and stroke or transient ischemic attack (TIA) were used to assess prevalence of CVD. If a participant reported either a heart condition or a stroke or TIA, they were classified as having CVD.
Covariates
Covariates included age (years), sex assigned at birth (female/male), race/ethnicity (non-Hispanic white, non-Hispanic black, or Hispanic), obesity (yes/no), current smoking status (yes/no), and DM or hyperglycemia (yes/no). Age, sex, and race/ethnicity were obtained from HRS self-reported demographic data. The presence of DM or hyperglycemia was obtained from HRS self-reported health data on the basis of a healthcare provider’s diagnosis. Smoking status was obtained from HRS self-reported health data. A participant was classified as obese if their calculated body mass index was ≥30 kg/m2, based on the widely recognized clinical classification of obesity (Jensen et al., 2014).
Statistical Analysis
Continuous variables were reported as weighted mean ± standard error, and categorical variables were reported as frequency (weighted percentage). Distributions of covariates by CVD diagnosis and risk score were reported using F-test for continuous variables and Rao-Scott Chi-Square Test for categorical variables. Weighted logistic regression models were used to calculate adjusted odds ratio (OR) of CVD in participants with low-, medium-, and high-risk groups. Model 1 was adjusted for age. Model 2 was adjusted for age, sex, race, obesity, smoking status, and DM.
All analyses were completed using SAS version 9.4 (SAS Institute, Inc., Cary, NC). P values at alpha < 0.05 were considered statistically significant.
Results
Descriptive statistics for those with and without CVD are provided in Table 1. The mean age of participants with CVD was 70.98 ± 0.56 years and 66.65 ± 0.33 years for participants without CVD (P < 0.05). Of the participants with CVD, 47.79% were female, and of the participants without CVD, 59.93% were female (P < 0.05). Distributions of race/ethnicity, obesity status, and smoking status were not significantly different between the two groups. There was a greater prevalence of DM or hyperglycemia in the CVD group. Mean LDL-C and HDL-C levels were significantly greater in the no CVD group, while there were no significant differences in mean TG or log CRP between the CVD and no CVD groups. HTN and high CRP were more prevalent in the CVD group, while dyslipidemia was more prevalent in the no CVD group.
Characteristics of HRS Participants with and without CVD (n = 1527)
Variables |
CVD (n = 470) |
No CVD (n = 1057) |
P Value |
|
|---|---|---|---|---|
Age (years) |
70.98 ± 0.56 |
66.65 ± 0.33 |
<0.0001 |
|
Sex (female), n (%) |
246 (47.79) |
653 (59.93) |
0.0006 |
|
Race/Ethnicity, n (%) |
Non-Hispanic white |
336 (82.30) |
691 (81.19) |
0.8809 |
Non-Hispanic black |
75 (8.82) |
188 (9.03) |
||
Hispanic |
59 (8.88) |
178 (9.78) |
||
Obesity, n (%) |
223 (47.30) |
461 (41.78) |
0.1211 |
|
Current Smoker, n (%) |
50 (8.82) |
110 (11.11) |
0.2768 |
|
DM or Hyperglycemia, n (%) |
182 (36.07) |
251 (20.25) |
<0.0001 |
|
LDL-C (mg/dL) |
94.26 ± 2.09 |
108.76 ± 1.38 |
<0.0001 |
|
HDL-C (mg/dL) |
51.97 ± 0.95 |
60.42 ± 0.81 |
<0.0001 |
|
TG (mg/dL) |
139.44 ± 3.98 |
137.27 ± 2.68 |
0.6503 |
|
Log CRP |
0.75 ± 0.07 |
0.59 ± 0.05 |
0.0698 |
|
HTN, n (%) |
375 (76.05) |
622 (54.42) |
<0.0001 |
|
Dyslipidemia, n (%) |
301 (65.19) |
795 (76.04) |
0.0006 |
|
High CRP, n (%) |
211 (42.27) |
405 (35.41) |
0.0470 |
n: number of participants, LDL-C: low-density lipoprotein cholesterol, mg/dL: milligrams per deciliter, HDL-C: high-density lipoprotein cholesterol, TG: triglyceride, CRP: C-reactive protein, HTN: hypertension.
Values are mean ± standard error and frequency (percentage); Bold = statistically significant at alpha < 0.05.
Distributions of covariates for low-, medium-, and high-risk factor scores based on presence of 0–1, 2, or 3 of HTN, dyslipidemia, and high CRP, respectively, are described in Table 2. The low-risk group mean age was 68.18 ± 0.49 years, the medium-risk group mean age was 67.70 ± 0.43 years, and the high-risk group mean age was 67.62 ± 0.60 years (P > 0.05). About 53.66%, 59.59%, and 56.17% of participants in the low-, medium-, and high-risk groups were female, respectively (P > 0.05). The proportion of non-Hispanic whites was highest in the low-risk group, while the medium and high-risk groups contained a greater proportion of non-Hispanic black participants and Hispanic participants compared to the low-risk group. The prevalence of obesity was patterned according to the risk group, with the highest average BMI in the high-risk group and lowest in the low-risk group. Smoking status was not significantly different between the three groups. There was an increasing prevalence of DM or hyperglycemia as the number of risk factors increased. Mean LDL-C, TG, and log CRP also increased with the increasing risk factor score, while mean HDL-C decreased.
Characteristics of HRS Participants by Risk Category (n = 1527)
Variables |
Low-Risk (n = 582) |
Medium-Risk (n = 601) |
High-Risk (n = 344) |
P Value |
|
|---|---|---|---|---|---|
Age (years) |
68.18 ± 0.49 |
67.70 ± 0.43 |
67.62 ± 0.60 |
0.7004 |
|
Sex (female), n (%) |
322 (53.66) |
364 (59.59) |
213 (56.17) |
0.2747 |
|
Race/Ethnicity, n (%) |
Non-Hispanic white |
436 (86.18) |
394 (80.09) |
197 (74.87) |
0.0006 |
Non-Hispanic black |
75 (6.73) |
101 (8.39) |
87 (14.53) |
||
Hispanic |
71 (7.09) |
106 (11.53) |
60 (10.60) |
||
Obesity, n (%) |
164 (25.67) |
296 (48.42) |
224 (68.95) |
<0.0001 |
|
Current Smoker, n (%) |
57 (8.64) |
56 (10.08) |
47 (14.79) |
0.0910 |
|
DM or Hyperglycemia, n (%) |
132 (19.32) |
171 (25.43) |
130 (34.32) |
0.0003 |
|
LDL-C (mg/dL) |
96.31 ± 1.70 |
109.38 ± 1.83 |
112.21 ± 2.78 |
<0.0001 |
|
HDL-C (mg/dL) |
64.61 ± 1.08 |
56.01 ± 0.97 |
48.66 ± 1.04 |
<0.0001 |
|
TG (mg/dL) |
114.88 ± 3.02 |
146.62 ± 3.33 |
167.17 ± 5.46 |
<0.0001 |
|
Log CRP |
0.09 ± 0.05 |
0.68 ± 0.06 |
1.66 ± 0.07 |
<0.0001 |
|
HTN, n (%) |
193 (28.11) |
460 (73.91) |
344 (100.00) |
||
Dyslipidemia, n (%) |
249 (46.08) |
503 (86.92) |
344 (100.00) |
||
High CRP, n (%) |
33 (4.17) |
239 (39.17) |
344 (100.00) |
n: number of participants, LDL-C: low-density lipoprotein cholesterol, mg/dL: milligrams per deciliter, HDL-C: high-density lipoprotein cholesterol, TG: triglyceride, CRP: C-reactive protein, HTN: hypertension.
Values are mean ± standard error and frequency (percentage); Bold = statistically significant at alpha < 0.05.
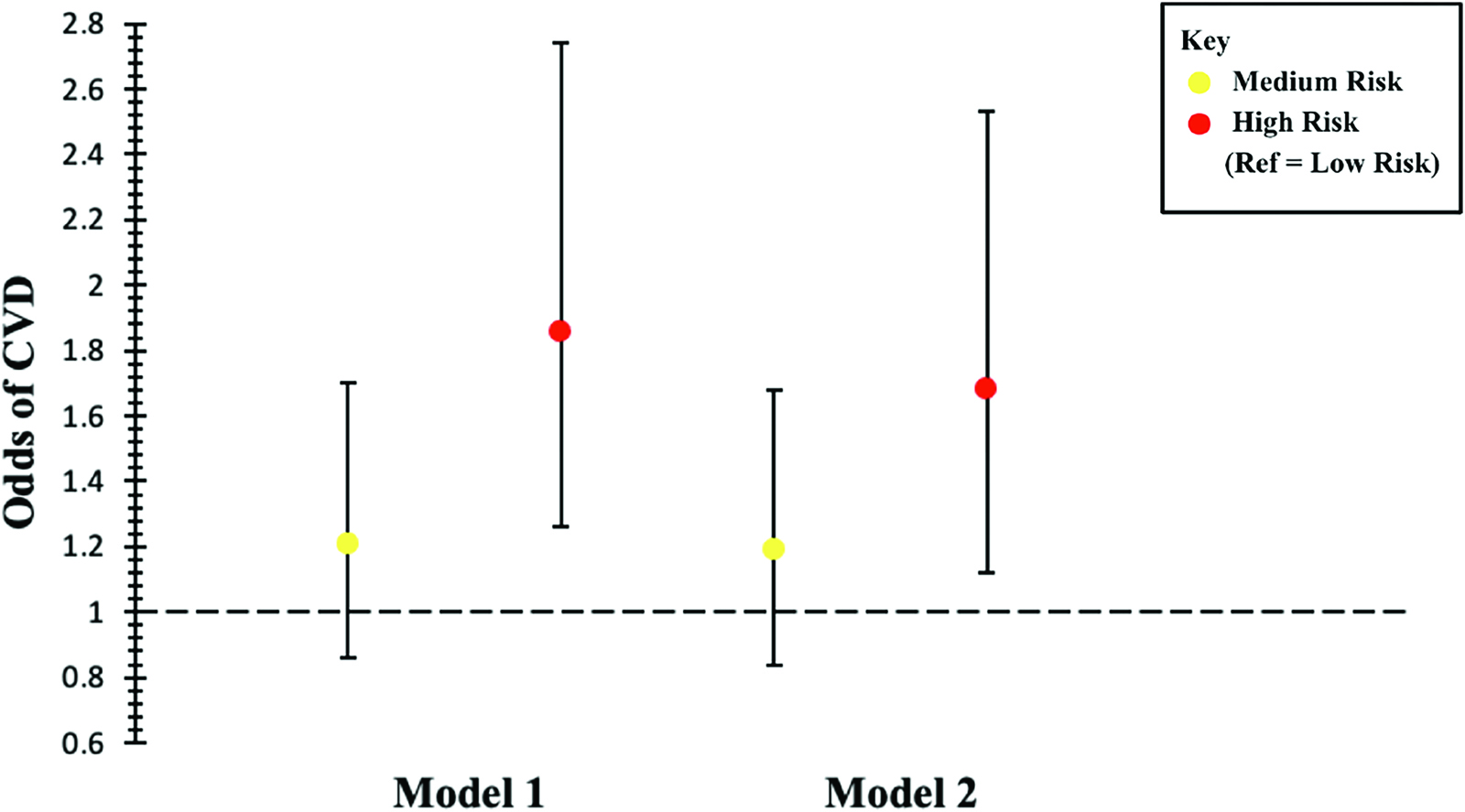
Associations between risk factor score and CVD prevalence are shown in Table 3 and Figure 1. The prevalence of CVD in medium-risk participants was not significantly different compared to low-risk participants. After adjusting for age, the odds of CVD prevalence in high-risk participants were 1.86-fold higher (95% CI: [1.26–2.74]) compared to low-risk participants. This association was robust to additional adjustment for sex, race/ethnicity, obesity status, smoking status, and DM or hyperglycemia status (OR = 1.68, 95% CI: [1.12–2.53]).
Adjusted Odds of CVD Prevalence for Risk Category in HRS Participants (n = 1527)
Low-Risk |
Medium-Risk |
High-Risk |
|
|---|---|---|---|
OR (95% CI) |
|||
Model 1 |
Ref |
1.21 (0.86–1.70) |
1.86 (1.26–2.74) |
Model 2 |
Ref |
1.19 (0.84–1.68) |
1.68 (1.12–2.53) |
OR: odds ratio, CI: confidence interval.
Model 1: Adjusted for age.
Model 2: Adjusted for age, sex, race/ethnicity, obesity, current smoker, DM, or hyperglycemia.
Discussion
HTN and dyslipidemia are well established as contributing factors in the development of CVD. More recently, elevated inflammatory status, measured through elevated CRP, has been explored as an important element in determining the risk of CVD. This study investigated the prevalence of CVD between groups with 0–1, 2, or 3 of these conditions in a nationally representative sample of US adults over the age of 50.
Our findings revealed that those with 0–1 or 2 risk factors all had similar prevalence of CVD, but those with all 3 CVD risk factors had a significantly higher prevalence of CVD, even after adjusting for age, sex, race/ethnicity, obesity status, smoking status, and presence of DM or hyperglycemia. This supports the hypothesis that CVD is multifactorial, and the combination of HTN, dyslipidemia, and inflammation is characteristic of those with healthcare provider–diagnosed CVD. As a result, screening for all 3 factors may be clinically important for identifying those at the risk of developing CVD.
These results are consistent with previous research, including multiple observational and experimental studies. Previously, based on the traditional cholesterol hypothesis of CVD, which places the blame of atherosclerosis primarily on cholesterol, many medications were produced with the goal of reducing cholesterol. One promising medication, niacin, resulted in significant improvements in participant lipid profiles, including lowered TG and LDL-C and greater HDL-C. Despite these improvements in lipids, participants demonstrated zero clinical benefit, and some even had worse CVD outcomes (AIM-HIGH Investigators, 2011). In addition, even on multidrug lipid-lowering therapy, 1 in 5 participants experience residual CVD risk (Alfaddagh et al., 2020; Sampson et al., 2011). In multiple CVD drug trials, including PROVE-IT and IMPROVE-IT, residual CVD risk was associated with CRP elevation despite achieving control of cholesterol (Ridker et al., 2005; Bohula et al., 2015). Other CVD drug trials, such as JUPITER and CANTOS, have found that the anti- inflammatory and CRP-lowering effects of drugs, including statins, produce a protective effect against CVD independent of cholesterol-lowering effects (Willerson & Ridker, 2004; Ridker et al., 2009a; Ridker et al., 2009b; Ridker et al., 2017; Ridker et al., 2023). Our current knowledge has moved from believing that CVD stemmed from cholesterol buildup alone to a multifaceted disease, cholesterol being just one piece of the ever-changing puzzle that is the human body. HTN and dyslipidemia are the most significant players in the development of CVD, but studies have started to point toward inflammation as a component in the etiology of CVD as well. Inflammation is likely to be involved in atherosclerosis, and determining CVD risk based on a plethora of factors, not just BP and cholesterol, is prudent in the mitigation of CVD-related deaths. In practice, proper and comprehensive assessment of BP, lipids, and CRP is rare, which is especially problematic in CVD, a multifactorial condition. In this context, it is important to ensure implementation of current diagnostic guidelines, which advocate for concurrent and routine BP and lipid measurements in all adults, as well as CRP measurements in individuals at the risk of CVD, because just knowing one or two of these health parameters is not enough to accurately describe CVD risk (Pearson et al., 2003).
Overall, participants with CVD tended to be older and were more likely to be male and to have DM or hyperglycemia, as expected. In addition, participants with CVD had lower LDL-C and HDL-C levels, were more likely to have HTN and elevated CRP, and were less likely to be dyslipidemic. The lower prevalence of dyslipidemia in those with CVD could be due to lipid-lowering medication use, which is a first-line agent in the prevention of future CVD events in those previously diagnosed with CVD. Thorough analysis of medication usage is beyond the scope of this paper but is an area for future research. We found that non-Hispanic black and Hispanic participants made up a greater proportion of the high-risk category than the low-risk category, and vice versa, for non- Hispanic white participants. In addition, high-risk participants were more likely to be obese and to have DM or hyperglycemia.
This study has many strengths. Data come from HRS, a large and representative sample of older US adults. Measures of key laboratory variables, including CRP and lipids, were obtained using a standard protocol. HRS contains a wealth of information on covariates, allowing the analyses to be controlled for potential confounders of the association. Limitations of this study include the absence of longitudinal hypertensive, lipid, inflammatory, and cardiovascular data. An analysis of this longitudinal data would provide insight into the temporal order of CVD risk factors and CVD and thus can support causal relationships between these variables. In addition, CVD, obesity, smoking status, DM or hyperglycemia, and HTN are all self-reported from participants, and these data can be influenced by memory, social desirability bias, and absence of diagnosis. Smoking status and obesity are highly variable across individuals, and with a yes/no metric, it is difficult to ascertain the severity of these variables (i.e., how many pack-years and overall BMI). Other lifestyle information, such as physical activity, diet, and stress, which are all independent factors in development of CVD (Osborne et al., 2020; Li & Siegrist, 2012; Casas et al., 2018), was not included as covariates. Furthermore, pharmacological interventions are not analyzed, as it is outside the scope of this study. Finally, biomarkers that have been shown to more accurately predict CVD risk, such as LDL particle size, Lipoprotein(a), and Apolipoprotein B, were not available for analysis in HRS (Lamarche et al., 1997; Behbodikhah et al., 2021; Duarte Lau & Giugliano, 2022). Future studies should focus on implementing a variety of lifestyle factors and above-mentioned biomarkers on top of the CVD risk factors examined in this study to develop a comprehensive score of CVD risk.
Conclusion
The co-occurrence of HTN, dyslipidemia, and elevated CRP was associated with CVD prevalence in a representative sample of older US adults. Our findings emphasize the importance of multifactor screening for assessing CVD risk in the clinical setting.
Abbreviations
CVDcardiovascular disease
USUnited States
CADcoronary artery disease
CHDcoronary heart disease
PADperipheral artery disease
HTNhypertension
DMdiabetes mellitus
SBPsystolic blood pressure
DBPdiastolic blood pressure
mmHgmillimeters of mercury
TCtotal cholesterol
LDL-Clow-density lipoprotein cholesterol
HDL-Chigh-density lipoprotein cholesterol
TGtriglyceride
CRPC-reactive protein
nnumber of participants
mg/dLmilligrams per deciliter
HRSHealth and Retirement Study
NIANational Institute on Aging
ISRInstitute for Social Research
SSASocial Security Administration
TCtotal cholesterol
ORodds ratio
CIconfidence interval
References
Adukauskienė, D., Čiginskienė, A., Adukauskaitė, A., Pentiokinienė, D., Šlapikas, R., & Čeponienė, I. (2016). Clinical relevance of high sensitivity C-reactive protein in Cardiology. Medicina, 52(1), 1–10. https://doi.org/10.1016/j.medici.2015.12.001https://doi.org/10.1016/j.medici.2015.12.001
AIM-HIGH Investigators, Boden, W. E., Probstfield, J. L., Anderson, T., Chaitman, B. R., Desvignes-Nickens, P., Koprowicz, K., McBride, R., Teo, K., & Weintraub, W. (2011). Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. The New England Journal of Medicine, 365(24), 2255–2267. https://doi.org/10.1056/NEJMoa1107579https://doi.org/10.1056/NEJMoa1107579
Alfaddagh, A., Martin, S. S., Leucker, T. M., Michos, E. D., Blaha, M. J., Lowenstein, C. J., Jones, S. R., & Toth, P. P. (2020). Inflammation and cardiovascular disease: From mechanisms to therapeutics. American Journal of Preventive Cardiology, 4, 100130. https://doi.org/10.1016/j.ajpc.2020.100130https://doi.org/10.1016/j.ajpc.2020.100130
Alloubani, A. (2021). Relationship between hyperlipidemia, cardiovascular disease and stroke: A systematic review. Current Cardiology Reviews, 17(6), 9–23. https://doi.org/10.2174/18756557mteypmzi4whttps://doi.org/10.2174/18756557mteypmzi4w
Behbodikhah, J., Ahmed, S., Elyasi, A., Kasselman, L. J., De Leon, J., Glass, A. D., & Reiss, A. B. (2021). Apolipoprotein B and cardiovascular disease: Biomarker and potential therapeutic target. Metabolites, 11(10), 690. https://doi.org/10.3390/metabo11100690https://doi.org/10.3390/metabo11100690
Benjamin, E. J., Virani, S. S., Callaway, C. W., Chamberlain, A. M., Chang, A. R., Cheng, S., Chiuve, S. E., Cushman, M., Delling, F. N., Deo, R., de Ferranti, S. D., Ferguson, J. F., Fornage, M., Gillespie, C., Isasi, C. R., Jiménez, M. C., Jordan, L. C., Judd, S. E., Lackland, D., … Muntner, P. (2018). Heart disease and stroke statistics — 2018 update: A report from the American Heart Association. Circulation, 137(12). https://doi.org/10.1161/cir.0000000000000558https://doi.org/10.1161/cir.0000000000000558
Bohula, E. A., Giugliano, R. P., Cannon, C. P., Zhou, J., Murphy, S. A., White, J. A., Tershakovec, A. M., Blazing, M. A., & Braunwald, E. (2015). Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of Ezetimibe to simvastatin and associated with better outcomes in improve-it. Circulation, 132(13), 1224–1233. https://doi.org/10.1161/circulationaha.115.018381https://doi.org/10.1161/circulationaha.115.018381
Carey, R. M., Wright, J. T., Taler, S. J., & Whelton, P. K. (2021). Guideline-driven management of hypertension. Circulation Research, 128(7), 827–846. https://doi.org/10.1161/circresaha.121.318083https://doi.org/10.1161/circresaha.121.318083
Casas, R., Castro-Barquero, S., Estruch, R., & Sacanella, E. (2018). Nutrition and cardiovascular health. International Journal of Molecular Sciences, 19(12), 3988. https://doi.org/10.3390/ijms19123988https://doi.org/10.3390/ijms19123988
Castro, A. R., Silva, S. O., & Soares, S. C. (2018). The use of high sensitivity C-reactive protein in cardiovascular disease detection. Journal of Pharmacy & Pharmaceutical Sciences, 21, 496–503. https://doi.org/10.18433/jpps29872https://doi.org/10.18433/jpps29872
Centers for Disease Control and Prevention. (2022, September 8). Heart disease and stroke. Centers for Disease Control and Prevention. Retrieved March 8, 2023, from https://www.cdc.gov/chronicdisease/resources/publications/factsheets/heart-disease-stroke.htmhttps://www.cdc.gov/chronicdisease/resources/publications/factsheets/heart-disease-stroke.htm
Centers for Disease Control and Prevention. (2023, January 18). FASTSTATS - leading causes of death. Centers for Disease Control and Prevention. Retrieved March 8, 2023, from https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htmhttps://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L. (2017). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204–7218. https://doi.org/10.18632/oncotarget.23208https://doi.org/10.18632/oncotarget.23208
Chen, T., Liu, G., & Yu, B. (2022). Colchicine for coronary artery disease: A Review. Frontiers in Cardiovascular Medicine, 9. https://doi.org/10.3389/fcvm.2022.892588https://doi.org/10.3389/fcvm.2022.892588
Davies, M. J., Woolf, N., Rowles, P. M., & Pepper, J. (1988). Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. Heart, 60(6), 459–464. https://doi.org/10.1136/hrt.60.6.459https://doi.org/10.1136/hrt.60.6.459
Duarte Lau, F., & Giugliano, R. P. (2022). Lipoprotein(a) and its significance in cardiovascular disease. JAMA Cardiology, 7(7), 760. https://doi.org/10.1001/jamacardio.2022.0987https://doi.org/10.1001/jamacardio.2022.0987
Farley, A., McLafferty, E., & Hendry, C. (2012). The cardiovascular system. Nursing Standard, 27(9), 35–39. https://doi.org/10.7748/ns2012.10.27.9.35.c9383https://doi.org/10.7748/ns2012.10.27.9.35.c9383
Ferrucci, L., & Fabbri, E. (2018). Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology, 15(9), 505–522. https://doi.org/10.1038/s41569-018-0064-2https://doi.org/10.1038/s41569-018-0064-2
Flora, G. D., & Nayak, M. K. (2019). A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Current Pharmaceutical Design, 25(38), 4063–4084. https://doi.org/10.2174/1381612825666190925163827https://doi.org/10.2174/1381612825666190925163827
Iqbal AM, Jamal SF. Essential Hypertension. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539859/https://www.ncbi.nlm.nih.gov/books/NBK539859/
Jensen, M. D., Ryan, D. H., Apovian, C. M., Ard, J. D., Comuzzie, A. G., Donato, K. A., Hu, F. B., Hubbard, V. S., Jakicic, J. M., Kushner, R. F., Loria, C. M., Millen, B. E., Nonas, C. A., Pi-Sunyer, F. X., Stevens, J., Stevens, V. J., Wadden, T. A., Wolfe, B. M., & Yanovski, S. Z. (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation, 129(25_suppl_2). https://doi.org/10.1161/01.cir.0000437739.71477.eehttps://doi.org/10.1161/01.cir.0000437739.71477.ee
Johnson, K. W., Dudley, J. T., & Bobe, J. R. (2018). A 72-year-old patient with longstanding, untreated familial hypercholesterolemia but no coronary artery calcification: A case report. Cureus. https://doi.org/10.7759/cureus.2452https://doi.org/10.7759/cureus.2452
Jung, E., Kong, S. Y., Ro, Y. S., Ryu, H. H., & Shin, S. D. (2022). Serum cholesterol levels and risk of CARDIOVASCULAR DEATH: A systematic review and a dose-response meta-analysis of prospective cohort studies. International Journal of Environmental Research and Public Health, 19(14), 8272. https://doi.org/10.3390/ijerph19148272https://doi.org/10.3390/ijerph19148272
Juster, F. T., & Suzman, R. (1993). The Health and Retirement Study: An Overview. National Institute on Aging, National Institutes of Health.
Karp, F. (2007). Growing older in America the health & retirement study. National Institute on Aging, National Institutes of Health, U.S. Dept. of Health and Human Services.
Khot, U. N. (2003). Prevalence of conventional risk factors in patients with coronary heart disease. JAMA, 290(7), 898. https://doi.org/10.1001/jama.290.7.898https://doi.org/10.1001/jama.290.7.898
Koosha, P., Roohafza, H., Sarrafzadegan, N., Vakhshoori, M., Talaei, M., Sheikhbahaei, E., & Sadeghi, M. (2020). High sensitivity C-reactive protein predictive value for cardiovascular disease: A nested case control from Isfahan Cohort Study (ICS). Global Heart, 15(1), 3. https://doi.org/10.5334/gh.367https://doi.org/10.5334/gh.367
Lamarche, B., Tchernof, A., Moorjani, S., Cantin, B., Dagenais, G. R., Lupien, P. J., & Despre´s, J.-P. (1997). Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Circulation, 95(1), 69–75. https://doi.org/10.1161/01.cir.95.1.69https://doi.org/10.1161/01.cir.95.1.69
Li, J., & Siegrist, J. (2012). Physical activity and risk of cardiovascular disease — a meta-analysis of prospective cohort studies. International Journal of Environmental Research and Public Health, 9(2), 391–407. https://doi.org/10.3390/ijerph9020391https://doi.org/10.3390/ijerph9020391
Libby, P., Ridker, P. M., & Hansson, G. K. (2011). Progress and challenges in translating the biology of atherosclerosis. Nature, 473(7347), 317–325. https://doi.org/10.1038/nature10146https://doi.org/10.1038/nature10146
Libby, P., Ridker, P. M., & Maseri, A. (2002). Inflammation and atherosclerosis. Circulation, 105(9), 1135–1143. https://doi.org/10.1161/hc0902.104353https://doi.org/10.1161/hc0902.104353
Lopez, A. D. (2006). Global burden of disease and risk factors. Oxford University Press.
Martin, S. S., & Cardoso, R. (2021, July 16). Hypercholesterolemia. Hypercholesterolemia - Symptoms, Causes, Images, and Treatment Options. Retrieved March 8, 2023, from https://online.epocrates.com/diseases/170/hypercholesterolemiahttps://online.epocrates.com/diseases/170/hypercholesterolemia
Martinez-Quinones, P., McCarthy, C. G., Watts, S. W., Klee, N. S., Komic, A., Calmasini, F. B., Priviero, F., Warner, A., Chenghao, Y., & Wenceslau, C. F. (2018). Hypertension induced morphological and physiological changes in cells of the arterial wall. American Journal of Hypertension, 31(10), 1067–1078. https://doi.org/10.1093/ajh/hpy083https://doi.org/10.1093/ajh/hpy083
Osborne, M. T., Shin, L. M., Mehta, N. N., Pitman, R. K., Fayad, Z. A., & Tawakol, A. (2020). Disentangling the links between Psychosocial Stress and cardiovascular disease. Circulation: Cardiovascular Imaging, 13(8). https://doi.org/10.1161/circimaging.120.010931https://doi.org/10.1161/circimaging.120.010931
Pearson, T. A., Mensah, G. A., Alexander, R. W., Anderson, J. L., Cannon, R. O., Criqui, M., Fadl, Y. Y., Fortmann, S. P., Hong, Y., Myers, G. L., Rifai, N., Smith, S. C., Taubert, K., Tracy, R. P., & Vinicor, F. (2003). Markers of inflammation and cardiovascular disease. Circulation, 107(3), 499–511. https://doi.org/10.1161/01.cir.0000052939.59093.45https://doi.org/10.1161/01.cir.0000052939.59093.45
Rhoads, J. P., & Major, A. S. (2018). How oxidized low-density lipoprotein activates inflammatory responses. Critical Reviews in Immunology, 38(4), 333–342. https://doi.org/10.1615/critrevimmunol.2018026483https://doi.org/10.1615/critrevimmunol.2018026483
Ridker, P. M., Brown, N. J., Vaughan, D. E., Harrison, D. G., & Mehta, J. L. (2004). Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation, 109(25_suppl_1). https://doi.org/10.1161/01.cir.0000133444.17867.56https://doi.org/10.1161/01.cir.0000133444.17867.56
Ridker, P. M., Cannon, C. P., & Morrow, D. (2005). C-reactive protein levels and outcomes after statin therapy. Journal of Vascular Surgery, 41(4), 733. https://doi.org/10.1016/j.jvs.2005.01.030https://doi.org/10.1016/j.jvs.2005.01.030
Ridker, P. M., Danielson, E., & Fonseca, F. A. H. (2009). Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. Journal of Vascular Surgery, 49(2), 534. https://doi.org/10.1016/j.jvs.2008.12.037https://doi.org/10.1016/j.jvs.2008.12.037
Ridker, P. M., Danielson, E., Fonseca, F. A. H., Genest, J., Gotto, A. M., Kastelein, J. J. P., Koenig, W., Libby, P., Lorenzatti, A. J., MacFadyen, J. G., Nordestgaard, B. G., Shepherd, J., Willerson, J. T., & Glynn, R. J. (2009). Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the Jupiter trial. The Lancet, 373(9670), 1175–1182. https://doi.org/10.1016/s0140-6736(09)60447-5https://doi.org/10.1016/s0140-6736(09)60447-5
Ridker, P. M., Everett, B. M., Thuren, T., MacFadyen, J. G., Chang, W. H., Ballantyne, C., Fonseca, F., Nicolau, J., Koenig, W., Anker, S. D., Kastelein, J. J. P., Cornel, J. H., Pais, P., Pella, D., Genest, J., Cifkova, R., Lorenzatti, A., Forster, T., Kobalava, Z., … Glynn, R. J. (2017). Antiinflammatory therapy with canakinumab for atherosclerotic disease. New England Journal of Medicine, 377(12), 1119–1131. https://doi.org/10.1056/nejmoa1707914https://doi.org/10.1056/nejmoa1707914
Ridker, Paul M, Bhatt, D. L., Pradhan, A. D., Glynn, R. J., MacFadyen, J. G., & Nissen, S. E. (2023). Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: A collaborative analysis of three randomised trials. The Lancet, 401(10384), 1293–1301. https://doi.org/10.1016/s0140-6736(23)00215-5https://doi.org/10.1016/s0140-6736(23)00215-5
Roth, G. A., Mensah, G. A., Johnson, C. O., Addolorato, G., Ammirati, E., Baddour, L. M., Barengo, N. C., Beaton, A. Z., Benjamin, E. J., Benziger, C. P., Bonny, A., Brauer, M., Brodmann, M., Cahill, T. J., Carapetis, J., Catapano, A. L., Chugh, S. S., Cooper, L. T., Coresh, J., … Fuster, V. (2020). Global burden of cardiovascular diseases and risk factors, 1990–2019. Journal of the American College of Cardiology, 76(25), 2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010https://doi.org/10.1016/j.jacc.2020.11.010
Sampson, U. K., Fazio, S., & Linton, M. R. F. (2011). Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: The evidence, etiology, and therapeutic challenges. Current Atherosclerosis Reports, 14(1), 1–10. https://doi.org/10.1007/s11883-011-0219-7https://doi.org/10.1007/s11883-011-0219-7
Sonnega, A., Faul, J. D., Ofstedal, M. B., Langa, K. M., Phillips, J. W., & Weir, D. R. (2014). Cohort profile: The health and retirement study (HRS). International Journal of Epidemiology, 43(2), 576–585. https://doi.org/10.1093/ije/dyu067https://doi.org/10.1093/ije/dyu067
Sorriento, D., & Iaccarino, G. (2019). Inflammation and cardiovascular diseases: The most recent findings. International Journal of Molecular Sciences, 20(16), 3879. https://doi.org/10.3390/ijms20163879https://doi.org/10.3390/ijms20163879
Steinberg, D. (2009). The LDL modification hypothesis of atherogenesis: An update. Journal of Lipid Research, 50. https://doi.org/10.1194/jlr.r800087-jlr200https://doi.org/10.1194/jlr.r800087-jlr200
Tabas, I., Williams, K. J., & Borén Jan. (2007). Subendothelial lipoprotein retention as the initiating process in atherosclerosis. Circulation, 116(16), 1832–1844. https://doi.org/10.1161/circulationaha.106.676890https://doi.org/10.1161/circulationaha.106.676890
Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Alonso, A., Beaton, A. Z., Bittencourt, M. S., Boehme, A. K., Buxton, A. E., Carson, A. P., Commodore-Mensah, Y., Elkind, M. S. V., Evenson, K. R., Eze-Nliam, C., Ferguson, J. F., Generoso, G., Ho, J. E., Kalani, R., Khan, S. S., Kissela, B. M., … Martin, S. S. (2022). Heart disease and stroke statistics — 2022 update: A report from the American Heart Association. Circulation, 145(8). https://doi.org/10.1161/cir.0000000000001052https://doi.org/10.1161/cir.0000000000001052
Venous blood collection and assay protocol in the 2016 Health and Retirement Study. HRS. (n.d.). Retrieved March 8, 2023, from https://hrs.isr.umich.edu/publications/biblio/9065https://hrs.isr.umich.edu/publications/biblio/9065
Willerson, J. T., & Ridker, P. M. (2004). Inflammation as a cardiovascular risk factor. Circulation, 109(21_suppl_1). https://doi.org/10.1161/01.cir.0000129535.04194.38https://doi.org/10.1161/01.cir.0000129535.04194.38
Wilson, P. W., D’Agostino, R. B., Levy, D., Belanger, A. M., Silbershatz, H., & Kannel, W. B. (1998). Prediction of coronary heart disease using risk factor categories. Circulation, 97(18), 1837–1847. https://doi.org/10.1161/01.cir.97.18.1837https://doi.org/10.1161/01.cir.97.18.1837
World Economic Forum. (2011). The Global Economic Burden of Non-communicable diseases: A report.