Introduction
The Problem
Ms. Smith is a 48-year-old female on hemodialysis for end-stage renal disease (ESRD). She is a schoolteacher and mother of three. Her transplant surgeon calls about an available donor kidney and explains that the donor is young but “increased risk” due to previous intravenous (IV) drug use. There is an unlikely possibility of transmission of undetected human immunodeficiency virus (HIV) or hepatitis, but her surgeon still recommends that she accept the organ. Ms. Smith is unsure. She has struggled with dialysis and wants to return to her work and family but wonders how much of a return to normalcy she could have with HIV. Ms. Smith asks her surgeon whether she will remain at the top of the transplant list if she declines this “high-risk” kidney. Her surgeon informs her that she will. Ms. Smith declines the offer.
Over 100,000 individuals currently need a lifesaving organ transplant, yet only 39,719 received a transplant in 2019.1 Despite this persistent supply-demand discrepancy that defines the organ donor system,1,2 Ms. Smith’s scenario is common. Such “high-risk” kidneys—officially classified as “increased risk donor” (IRD) organs—are rejected at 1.5 times the rate of non-IRD kidneys.3 The IRD classification denotes donors who meet criteria associated with greater risk for contracting HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV), including IV drug users and persons with hemophilia, in an effort to reduce infectious disease transmission.4,5 With modern nucleic acid testing (NAT) for HIV and HCV, the risk of unintended transmission is low but cannot be eliminated.4 For patients and providers, weighing the risk of disease transmission relative to remaining on the waitlist is challenging and prone to bias, potentially leading to underutilization of IRD organs and lives lost.
Context: History of IRD in the United States
Amid growing concerns about HIV transmission following transplant surgery, the Centers for Disease Control and Prevention (CDC) announced the 1994 Public Health Service (PHS) guidelines for IRD organs.6 In 2013, these guidelines were expanded to include criteria for increased risk status for HBV and HCV.7
In the years since the original IRD designation, much has changed. IRD organ donations from drug overdose deaths have increased due to the US opioid epidemic, resulting in an increase of potentially usable organs.8 The incidence of HIV has decreased,9 while hepatitis incidence continues to increase,10 and effective pharmacologic treatments have transformed outcomes for both diseases. Advancements in pharmacology and NAT have also shortened the diagnostic “window” periods, when the virus is undetectable despite being present.7
IRD and Decision-Making: Statistics and Biases
The kidney offered to Ms. Smith was labeled IRD due to the donor’s previous IV drug use. Estimates for the risk to a recipient of a window period infection for HIV and HCV undetected by enzyme-linked immunoassay (ELISA) and NAT per 10,000 donors are detailed in Table 1 (recreated from the Organ Procurement and Transplantation Network report7). There is a 30-fold difference in the risk of HCV transmission between an organ donated from an IV drug user with a negative serology test (3%) and an incarcerated individual with the same negative test (<0.1%). Nonetheless, these scenarios carry the same IRD designation.
Estimated Infection Risk to Recipients During Window Period of Undetectable Virus Levels Despite the Virus Being Presenta
Risk to recipient per 10,000 donors |
HIV ELISA |
HIV NAT |
HCV ELISA |
HCV NAT |
|---|---|---|---|---|
Men who have sex with men |
10.2 (0.10%) |
4.2 (<0.1%) |
32.5 (0.33%) |
3.5 (<0.1%) |
IV drug users |
12.1 (0.12%) |
4.9 (<0.1%) |
300.6 (3%) |
32.4 (0.32%) |
Persons with hemophilia |
0.086 (<0.01%) |
0.035 (<0.01%) |
0.26 (<0.1%) |
0.027 (<0.01%) |
Commercial sex worker |
6.6 (<0.1%) |
2.7 (<0.1%) |
114.9 (1.2%) |
12.3 (0.12%) |
Sex with a partner in above categories |
0.7 (<0.1%) |
0.3 (<0.1%) |
114.9 (1.2%) |
12.3 (0.12%) |
Blood product exposure |
1.5 (<0.1%) |
0.6 (<0.1%) |
4 (<0.1%) |
0.4 (<0.1%) |
Incarceration |
2.3 (<0.1%) |
0.9 (<0.1%) |
7.2 (<0.1%) |
0.8 (<0.1%) |
aRecreated from the Organ Procurement and Transplantation Network report.7
Abbreviations: HIV, human immunodeficiency virus; ELISA, enzyme-linked immunoassay; NAT, nucleic acid testing; HCV, hepatitis C virus.
While an IRD organ may represent an initial increased risk, there are also corollary risks of remaining on the transplant list, such as contracting HCV while receiving hemodialysis or death from organ failure.5 In 2017, more than 6500 transplant candidates died while on the waitlist.1 Given that patient and graft survival following transplantation are equivalent between IRD and non-IRD organs, acceptance of IRD organs compared with remaining on the waitlist provides a significant long-term survival benefit.11,12
Current IRD guidelines leave each organ offer vulnerable to the biases of provider and patient. Transplant surgeons’ bias of transmission risk based on IRD criteria do not correspond with actual risk, leading to underutilization of IRD organs.13 Research has shown, however, that with more direct guidance on IRD use, surgeon utilization of IRD organs increases.14 Additionally, improved patient education regarding true risks can improve utilization.15,16 Enhanced knowledge and communication about IRD risk could improve acceptance of IRD organs and patient outcomes.
A Path Forward: The Best Case/Worst Case Toolkit
Ms. Smith’s decision to turn down this “high-risk” kidney is understandable. Research shows that ratios and percentages are difficult for patients and providers to comprehend during risk–benefit discussions.17 Physician–patient communication around high-stakes decisions requires more than presenting data; it requires effective communication of risks and benefits through the lens of the patient’s values. Clinicians need better strategies to engage in risk–benefit conversations with patients around IRD organ offers as no gold standard exists.
Best Case/Worst Case (BC/WC) is a decision-making tool developed at the University of Wisconsin that has been shown to improve the quality of perioperative conversations between surgeons and their patients18 as well as promote shared decision-making between nephrologists and their patients around dialysis initiation.19 BC/WC conceptualizes risk through figures and stories rather than abstract percentages and probabilities. The keys to BC/WC are communicating the seriousness of the patient’s condition gently but clearly; utilizing storytelling and narratives; and providing patients and their families with a simple visual aid to better depict their options.
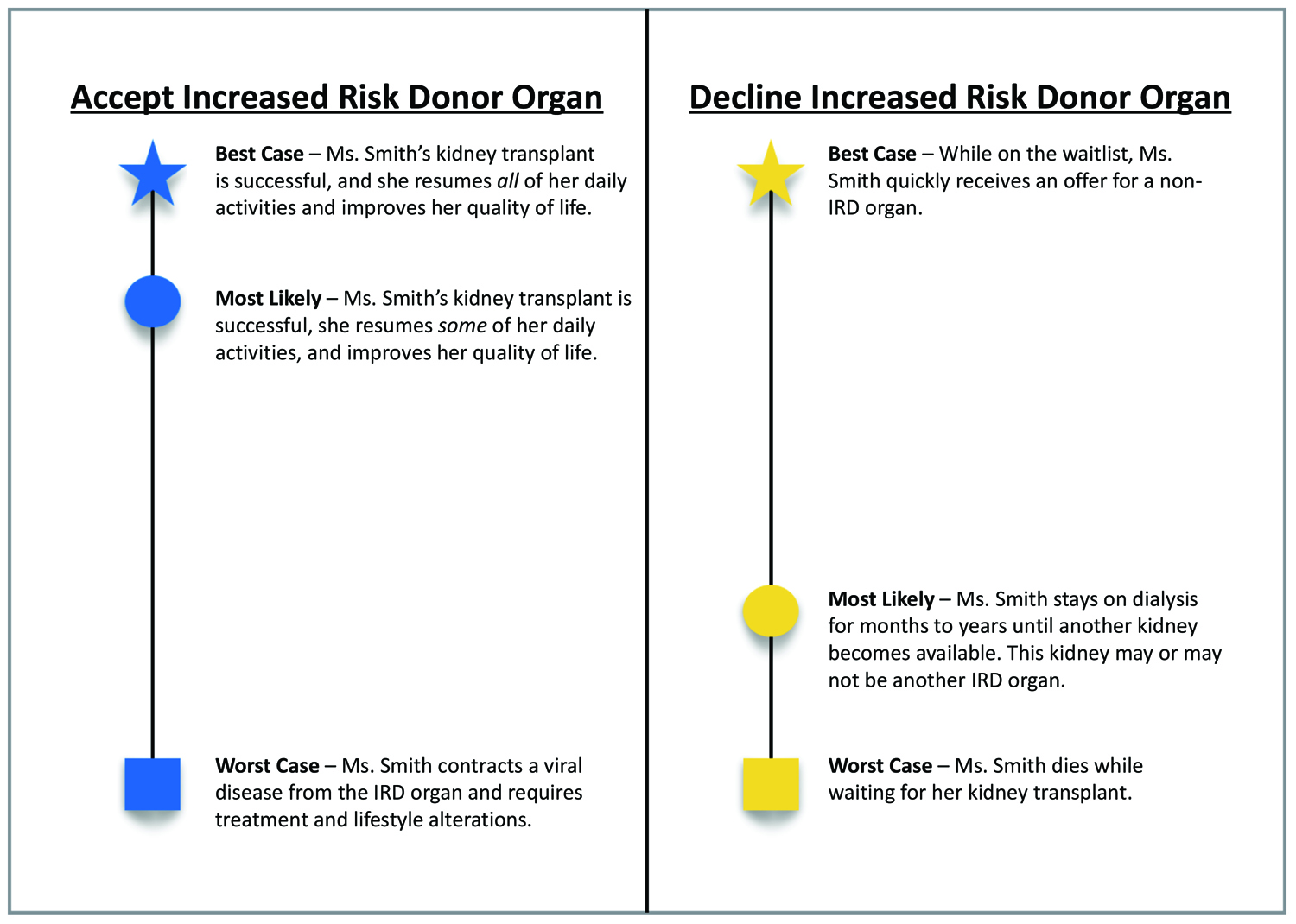
Although not yet validated in transplant surgery, we believe that BC/WC could foster improved provider–patient discussions around IRD organ offers. We present an example of how Ms. Smith’s transplant surgeon could utilize BC/WC to explain her two options—accepting the IRD kidney or remaining on the waitlist—and characterizing Ms. Smith’s “best case,” “worst case,” and most likely outcomes for each choice. We have adapted the BC/WC visual aid to further illustrate these options (Figure 1).
Best Case/Worst Case—Accepting the IRD Kidney
If Ms. Smith chooses to accept the IRD kidney, her Best Case outcome is a successful transplant. Her surgeon should describe the quality of life benefits, such as returning to caring for her family and work. This is also her Most Likely outcome.
In the Worst Case outcome, Ms. Smith contracts a viral disease from the IRD kidney. Her surgeon should describe the treatment regimen for each possible disease (e.g., lifetime antiretroviral therapy for HIV or near-guaranteed cure for HCV).
Best Case/Worst Case—Rejecting the IRD Kidney
If Ms. Smith instead chooses to remain on the waitlist, her Best Case outcome would be that she quickly receives an offer for a non-IRD kidney before her health declines further. Her surgeon should describe that she would remain on the waitlist and continue with dialysis but should also communicate that this scenario is unlikely.3,12
The Worst Case outcome of declining the IRD kidney is that Ms. Smith will die of ESRD complications while waiting for another kidney offer. Her surgeon can gently explain the expected decline in her quality of life and functional status.
In this situation, the Most Likely outcome of declining the IRD kidney would lie somewhere between the best and worst cases: she can most likely expect to wait some time for a new match. The graphical representation of probabilities in the visual aid is especially helpful, as Ms. Smith and her family can reference it after their discussion.
As Ms. Smith considers her options, her surgeon should elicit Ms. Smith’s values and goals as well as the risks that are acceptable to her. Using the BC/WC decision tool, Ms. Smith’s surgeon will help develop a shared understanding that accepting the IRD kidney would give her the most likely opportunity to resume the life that she values, but the ultimate decision is hers.
Conclusion: Suggestions for IRD Policy Change
Although limitations may exist for BC/WC regarding time constraints, provider training, and generalizability of the tool in various clinical contexts, the tool provides a useful framework to improve patient–provider shared decision-making around solid organ offers. In addition to BC/WC, more robust shared decision-making tools should also be developed to improve patient education around IRD organs. Future tools could include talking points for providers around IRD as well as answers to patients’ frequently asked questions. Prior to implementation, these tools should be validated in the clinical setting to ensure they meet the needs of patients and providers. These tools, once implemented, should be readily available in various languages, to ensure all patients have equal access to the shared decision-making process around IRD organ decisions. Hospital interpreters should also be trained in communicating the information included in these tools.
Broader policy change is also needed to update language in the PHS guidelines to better define IRD organ designation. The Department of Health and Human Services is currently revising recommendations for the PHS guidelines, with proposed changes such as altering the behavior timeframe and removing several criteria from the IRD designation.20 Given concerns that the current verbiage causes cognitive bias, recommendations have also been made to change the terminology of IRD.21 We strongly recommend such policy adjustments, considering the data suggest over 300 additional organ transplants could be performed annually in the absence of IRD status.9 Reflecting on the public health consequences of IRD organ underutilization, the transplant community must reevaluate whether the IRD label causes greater harm than benefit.
References
1. Transplant trends. United Network for Organ Sharing. Published 2019. Accessed July 2020. https://unos.org/data/transplant-trends/.https://unos.org/data/transplant-trends/
2. Harper AM, Rosendale JD, McBride MA, Cherikh WS, Ellison MD. The UNOS OPTN waiting list and donor registry. Clin Transpl. 1998:73–90.
3. Holscher CM, Bowring MG, Haugen CE, et al. National variation in increased infectious risk kidney offer acceptance. Transplantation. 2019; 103(10):2157–2163. doi:10.1097/TP.000000000000263110.1097/TP.0000000000002631
4. Seem DL, Lee I, Umscheid CA, Kuehnert MJ. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013; 128(4):247–343. doi:10.1177/00333549131280040310.1177/003335491312800403
5. OPTN/UNOS Ad-hoc Disease Transmission Advisory Committee. Guidance on Explaining Risk Related to Use of U.S. PHS Increased Risk Donor Organs When Considering Organ Offers: Public Comment Proposal. 2017. https://optn.transplant.hrsa.gov/media/2116/guidance_increased_risk_organ_offers_20170327.pdfhttps://optn.transplant.hrsa.gov/media/2116/guidance_increased_risk_organ_offers_20170327.pdf
6. Volk ML, Wilk AR, Wolfe C, Kaul DR. The “PHS Increased Risk” label is associated with nonutilization of hundreds of organs per year. Transplantation. 2017; 101(7):1666–1669. doi:10.1097/TP.000000000000167310.1097/TP.0000000000001673
7. Organ Procurement and Transplantation Network. Understanding the Risk of Transmission of HIV, Hepatitis B, and Hepatitis C from U.S. PHS Increased Risk Donors. 2017. https://optn.transplant.hrsa.gov/media/2270/dtac_guidance_risks_201706.pdfhttps://optn.transplant.hrsa.gov/media/2270/dtac_guidance_risks_201706.pdf
8. Torjesen I. Opioid epidemic leads to surge in US organ donors, study shows. BMJ. 2018;361. doi:10.1136/bmj.k172010.1136/bmj.k1720
9. Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008; 30(5):520–529. doi:10.1001/jama.300.5.52010.1001/jama.300.5.520
10. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance: United States, 2016. https://www.cdc.gov/hepatitis/statistics/2016surveillance/pdfs/2016hepsurveillancerpt.pdfhttps://www.cdc.gov/hepatitis/statistics/2016surveillance/pdfs/2016hepsurveillancerpt.pdf
11. Pruett TL, Clark MA, Taranto SE. Deceased organ donors and PHS risk identification: impact on organ usage and outcomes. Transplantation. 2017; 101(7):1670–1678. doi:10.1097/TP.000000000000171610.1097/TP.0000000000001716
12. Bowring MG, Holscher CM, Zhou S, et al. Turn down for what? Patient outcomes associated with declining increased infectious risk kidneys. Am J Transplant. 2018; 18(3):617–624. doi:10.1111/ajt.1457710.1111/ajt.14577
13. Gordon EJ, Mullee J, Beauvais N, et al. Education and informed consent about increased risk donor kidneys: a national survey of non-physician transplant providers. Transpl Infect Dis. 2014; 16(2):251–260. doi:10.1111/tid.1219910.1111/tid.12199
14. Kucirka LM, Namuyinga R, Hanrahan C, Montgomery RA, Segev DL. Formal policies and special informed consent are associated with higher provider utilization of CDC high-risk donor organs. Am J Transplant. 2009; 9(3):629–635. doi:10.1111/j.1600-6143.2008.02523.x10.1111/j.1600-6143.2008.02523.x
15. Humar SS, Liu J, Pinzon N, et al. Attitudes of liver transplant candidates toward organs from increased-risk donors. Liver Transplant. 2019; 25(6):881–888. doi:10.1002/lt.2546710.1002/lt.25467
16. Volk ML, Roney M, Fagerlin A. Pilot test of a patient decision aid about liver transplant organ quality. Liver Transplant. 2014; 20(7):850–855. doi:10.1002/lt.2388210.1002/lt.23882
17. Reyna VF, Nelson WL, Han PK, Dieckmann NF. How numeracy influences risk comprehension and medical decision making. Psychol Bull. 2009; 135(6):943–973. doi:10.1037/a001732710.1037/a0017327
18. Taylor LJ, Nabozny MJ, Steffens NM, et al. A framework to improve surgeon communication in high-stakes surgical decisions: Best Case/Worst Case. JAMA Surg. 2017; 152(6):531–538. doi:10.1001/jamasurg.2016.567410.1001/jamasurg.2016.5674
19. Zimmermann CJ, Jhagroo RA, Wakeen M, et al. Opportunities to improve shared decision making in dialysis decisions for older adults with life-limiting kidney disease: a pilot study. J Palliat Med. 2020; 23(5):627–634. doi:10.1089/jpm.2019.034010.1089/jpm.2019.0340
20. Department of Health and Human Services. Request for Information: Regarding Revisions to the PHS Guideline for Reducing Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV) Through Organ Transplantation. August 27, 2019. https://www.federalregister.gov/documents/2019/08/27/2019-17759/request-for-information-regarding-revisions-to-the-phs-guideline-for-reducing-human-immunodeficiencyhttps://www.federalregister.gov/documents/2019/08/27/2019-17759/request-for-information-regarding-revisions-to-the-phs-guideline-for-reducing-human-immunodeficiency
21. Advisory Committee on Blood and Tissue Safety and Availability. Formulation of committee findings and recommendations. In: ACBTSA April 16, 2019—Meeting Summary. Last reviewed May 17, 2019. https://www.hhs.gov/oidp/advisory-committee/blood-tissue-safety-availability/meeting-summary/2019-04-16/index.htmlhttps://www.hhs.gov/oidp/advisory-committee/blood-tissue-safety-availability/meeting-summary/2019-04-16/index.html